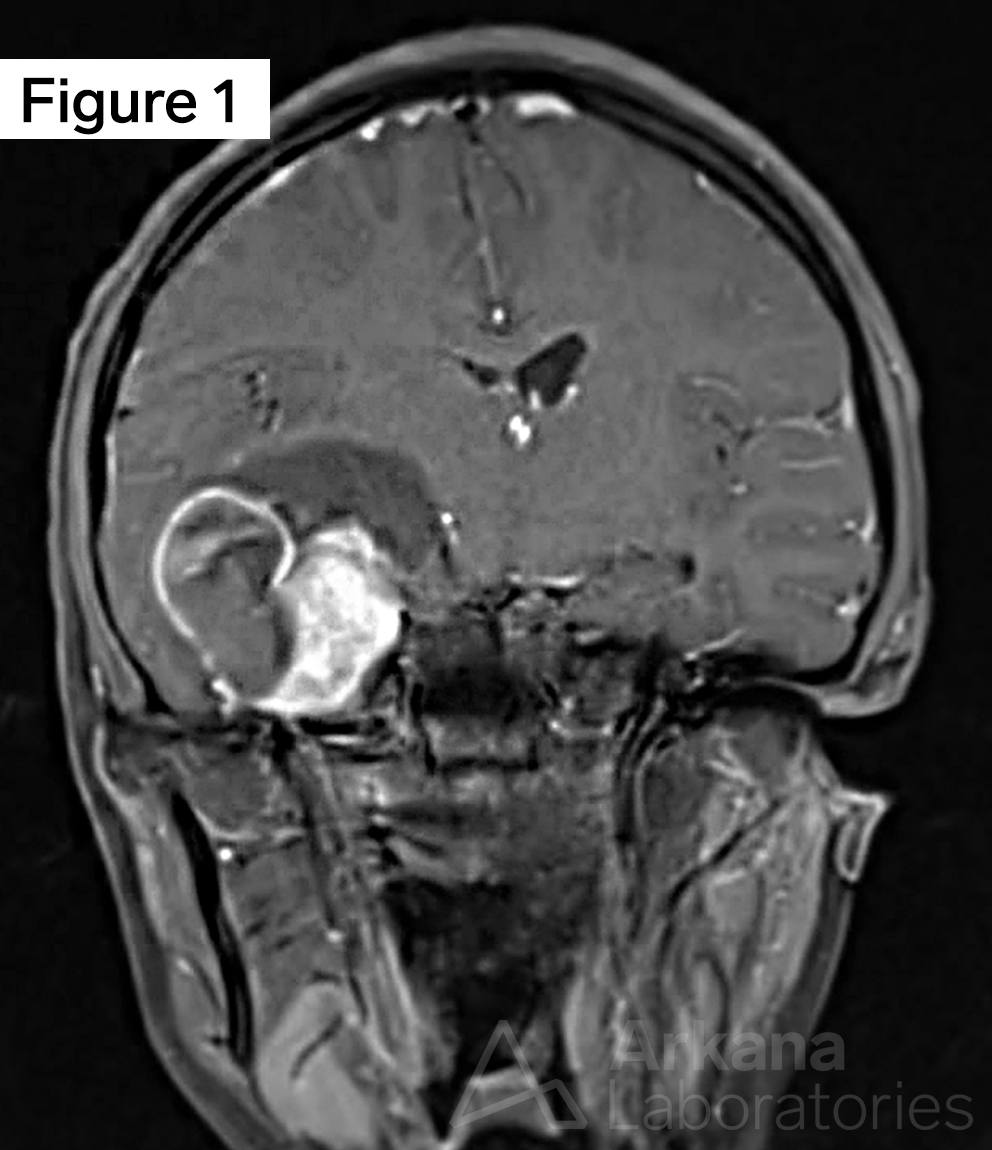
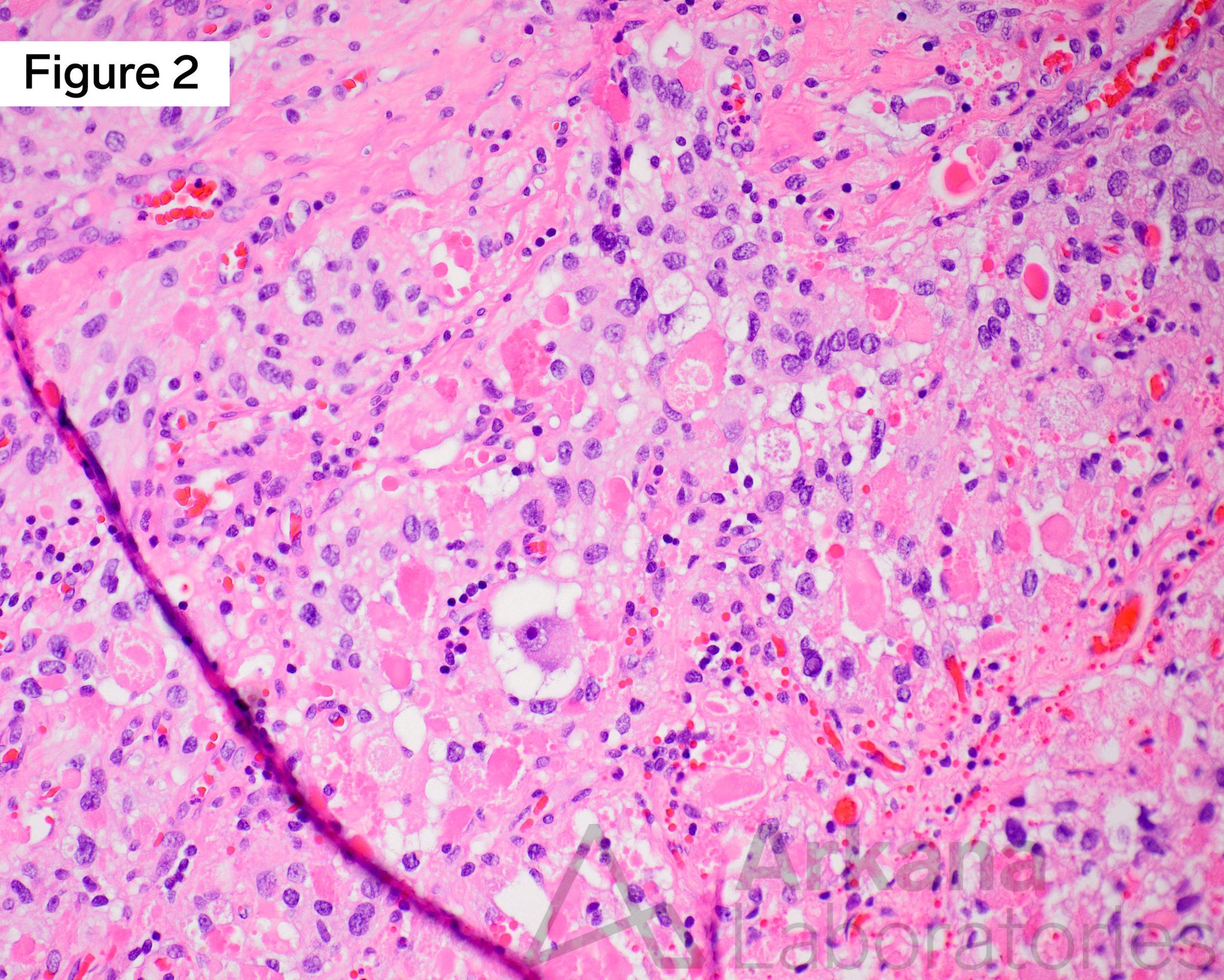
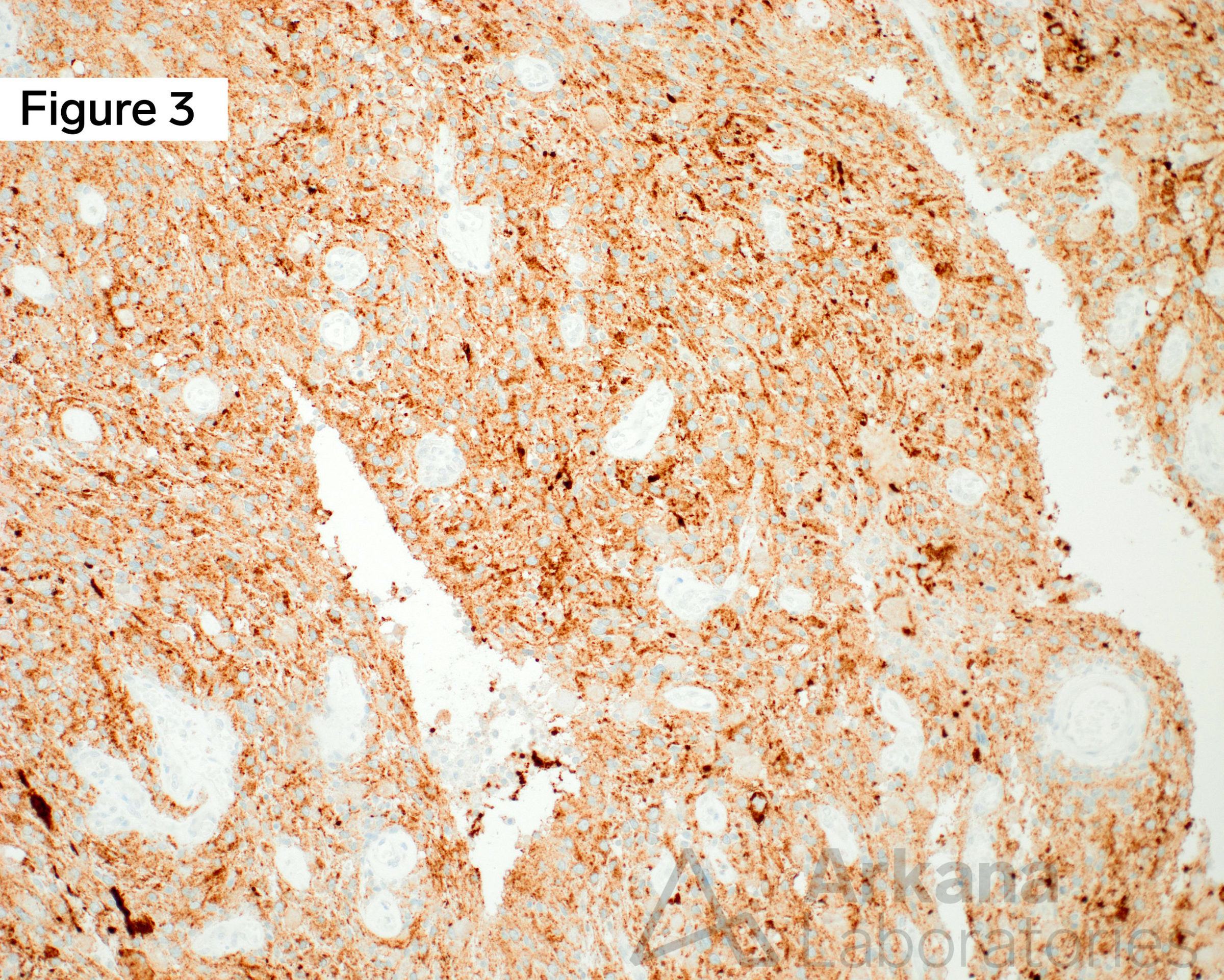
Continuing on from the clinical vignette last week, a young adult female presents with generalized headaches of several months duration. Neuroimaging with MRI Brain demonstrated a 4 cm multicystic mass with calcifications in the right temporal lobe. Using the figures above, what common genetic alteration may be assayed by immunostaining in this well-circumscribed low-grade cystic brain tumor?
A.IDH1 R132H
B.ATRX
C.INI1
D.BRAF V600E
Young adult female with a ganglioglioma (GG) in the right temporal lobe. Figure 1 is an MRI Brain image in the T1 sequence post-contrast in the coronal plane, which demonstrates a ~4 cm multicystic mass in the medial right temporal lobe with a solid mural nodular component with heterogeneous enhancement of the solid portions and cystic rim. Calcifications are noted along with the septal and wall components (not well demonstrated in the image shown). Figure 2 is a hematoxylin and eosin (H&E)-stained section of formalin-fixed paraffin-embedded (FFPE) tissue demonstrating a low-grade glioneuronal tumor containing a dysplastic ganglion cell with a background glioma component composed of delicate piloid astrocytes, reminiscent of pilocytic astrocytoma, as well as numerous eosinophilic granular bodies (EGBs). Figure 3 is an immunohistochemical (IHC) stain for BRAF V600E, which shows strong diffuse cytoplasmic immunoreactivity in the tumor cells. Original magnifications: A. MRI T1 Cor Post-Con; B. H&E stain, 400x; C. IHC stain for BRAF V600E, 100x.
Answer: BRAF V600E
This brain tumor is a classic example of a Ganglioglioma (GG), WHO grade I, BRAF V600E-mutated, answer choice (D).
Per the 2016 WHO Classification of CNS Tumors, revised 4th ed., gangliogliomas (GGs) are classified as mixed neuronal-glial tumors defined as well-differentiated slow-growing neoplasms composed of dysplastic ganglion cells in combination with neoplastic glial cells.
While a new CNS WHO edition will soon be available, the current CNS WHO states that the BRAF V600E is the most common genetic alteration in gangliogliomas (GGs), occurring in 20% to 60% of investigated cases.
Knowledge of BRAF V600E status may be helpful to guide targeted therapy drug selection (i.e. vemurafenib), especially in cases where the tumor is recurrent and/or in eloquent brain and gross total resection (GTR) is not possible.
In addition, a recent study by Pekmezci et al. explains that genetic alterations gangliogliomas are those that activate the MAP kinase pathway, and notes that a small subset of cases may also demonstrate CDKN2A deletion. See references.
Why were the other answers wrong?
Immunostains for IDH1 R132H and ATRX, answer choices (A) and (B) respectively, are essential in the work-up of diffuse astrocytomas.
Nuclear immunoreactivity for INI1 (SMARCB1), answer choice (C), a protein involved in chromatin remodeling, will be loss in Atypical Teratoid / Rhabdoid Tumor (AT/RT), an embryonal tumor of the CNS typically seen in pediatric patients.
References
Becker AJ, et al. Ganglioglioma. In: WHO classification of tumours in the central nervous system, 2016 revised 4th ed. Edited by DN Louis, et al. IARC Press, Lyon, France. 2016: 138-141.
del Bufalo F, Carai A, Figà-Talamanca L, et al. Response of recurrent BRAFV600E mutated ganglioglioma to Vemurafenib as single agent. J Transl Med. 2014 Dec 19;12:356. PMID: 25524464.
Pekmezci M, Villanueva-Meyer JE, Goode B, et al. The genetic landscape of ganglioglioma. Acta Neuropathol Commun. 2018 Jun 7;6(1):47. PMID: 29880043.
Schindler G, Capper D, Meyer J, et al. Analysis of BRAF V600E mutation in 1,320 nervous system tumors reveals high mutation frequencies in pleomorphic xanthoastrocytoma, ganglioglioma and extra-cerebellar pilocytic astrocytoma. Acta Neuropathol. 2011 Mar;121(3):397-405. PMID: 21274720.
Quick note: This post is to be used for informational purposes only and does not constitute medical or health advice. Each person should consult their own doctor with respect to matters referenced. Arkana Laboratories assumes no liability for actions taken in reliance upon the information contained herein.