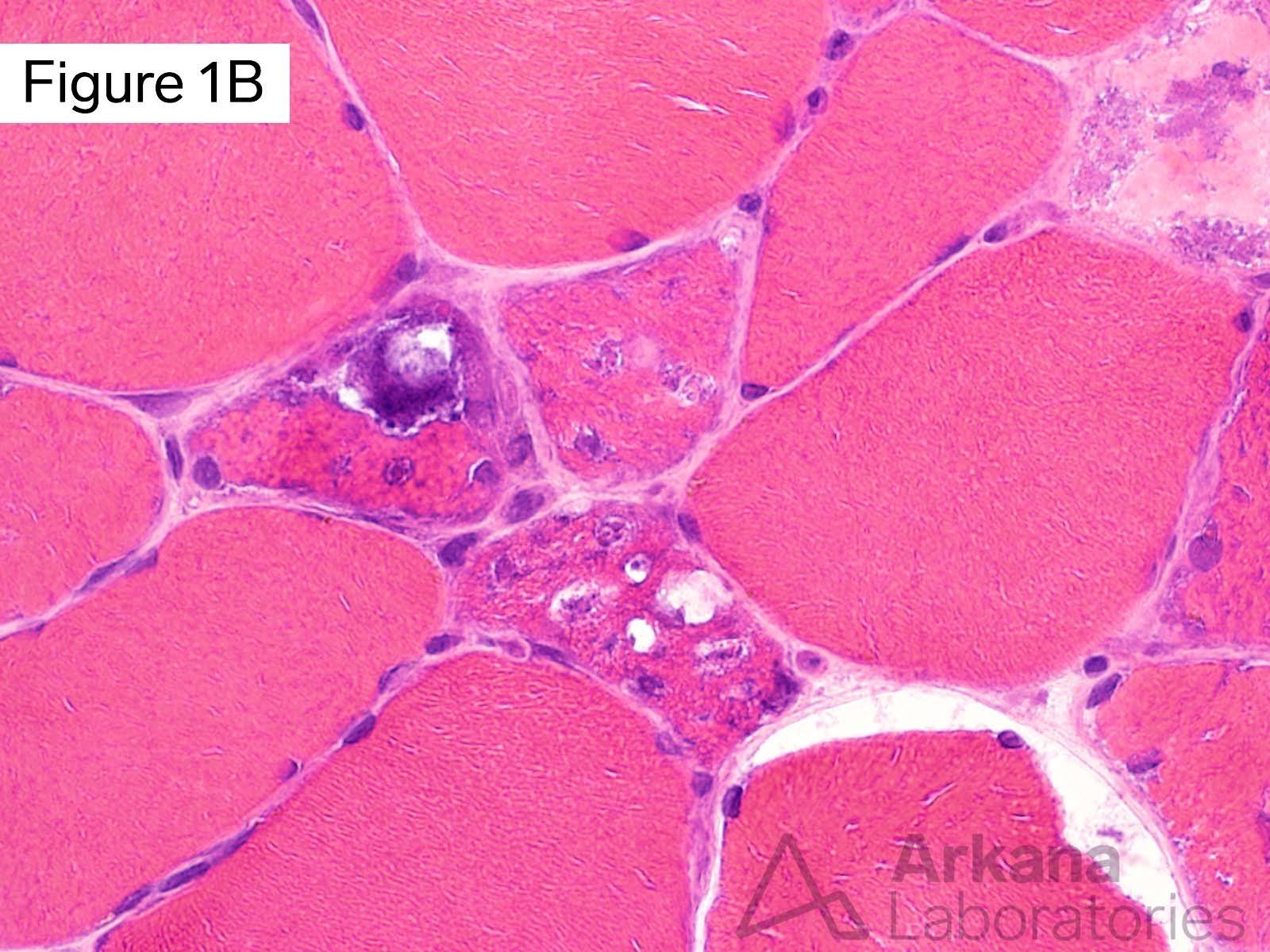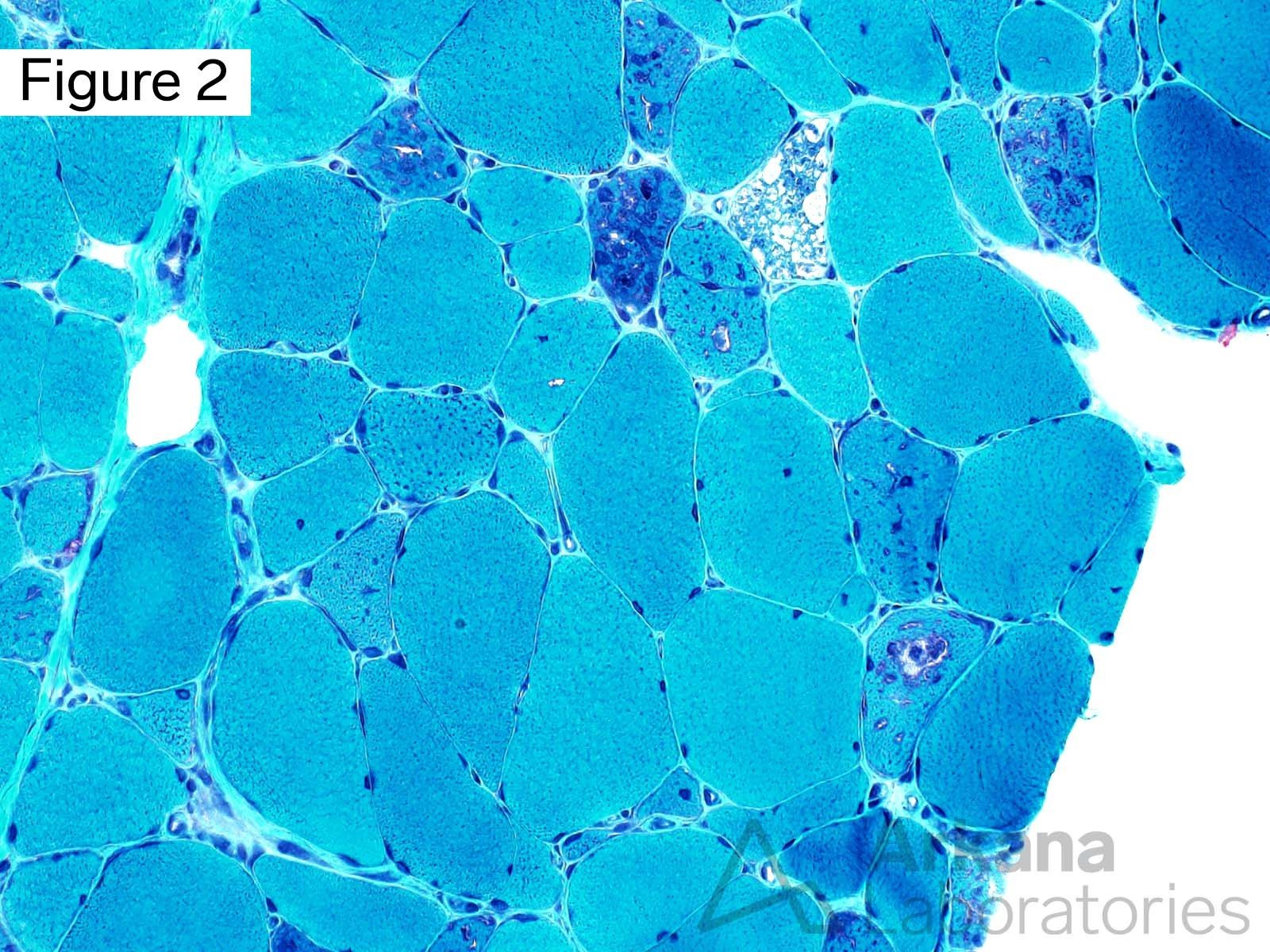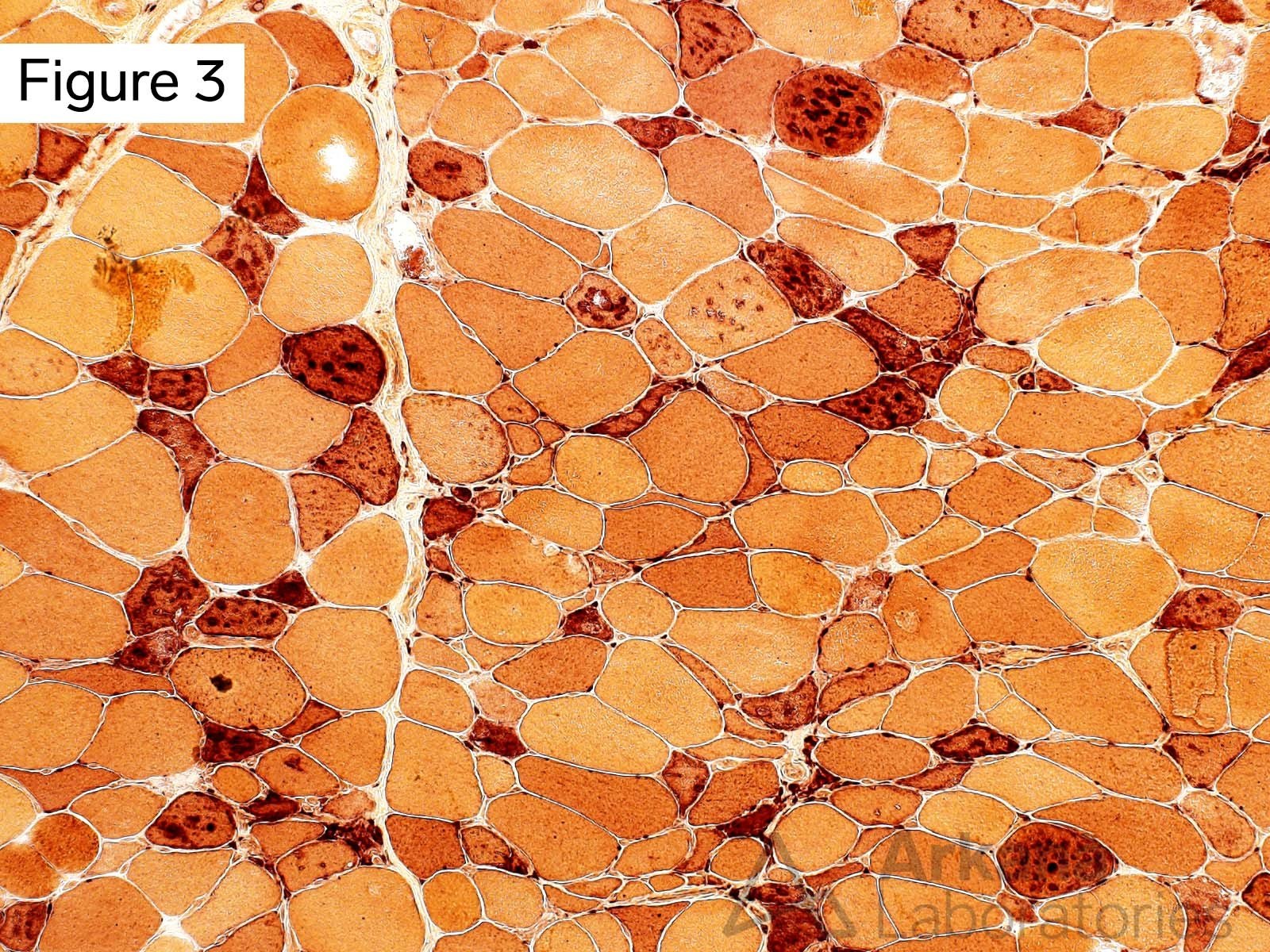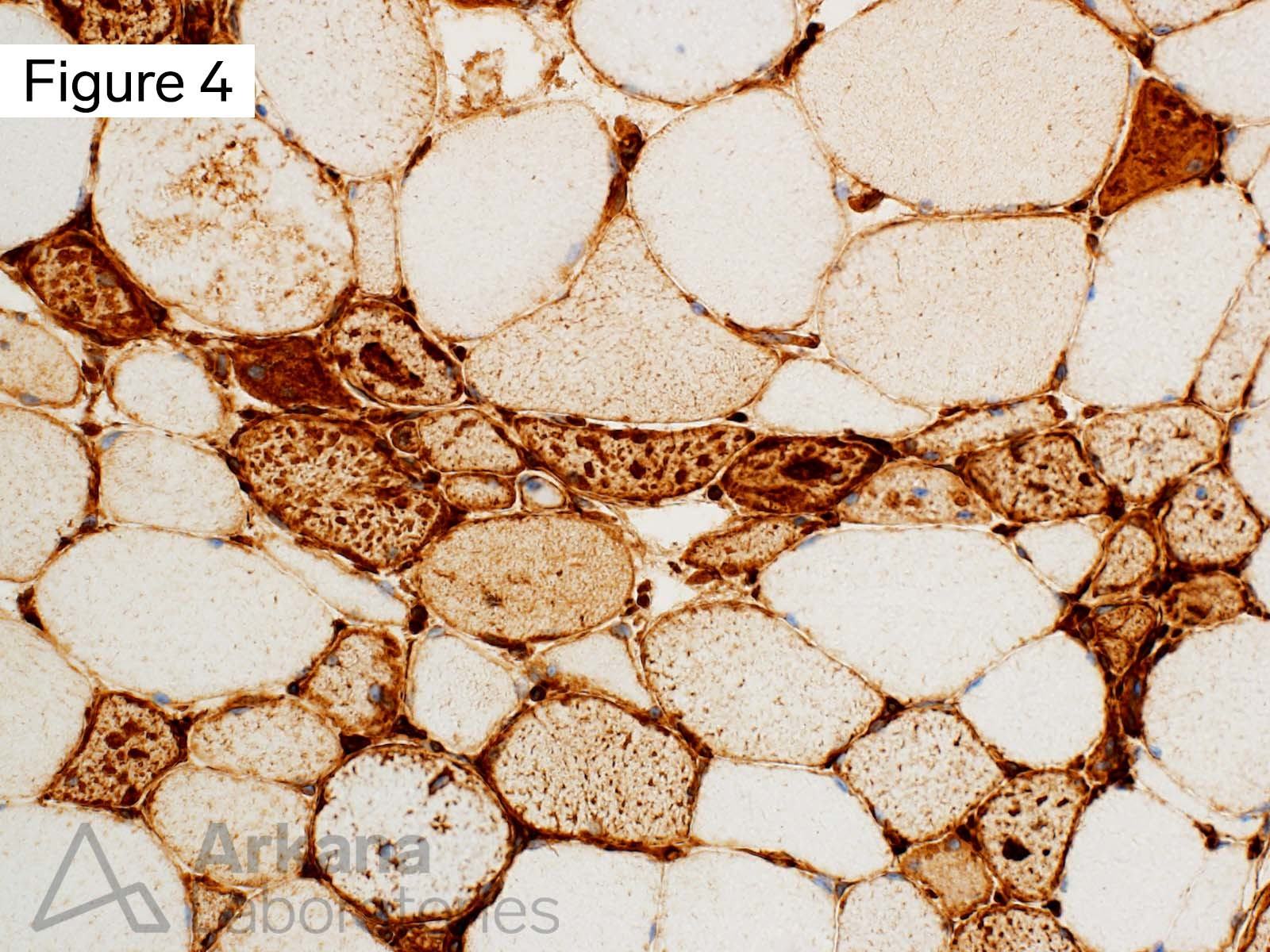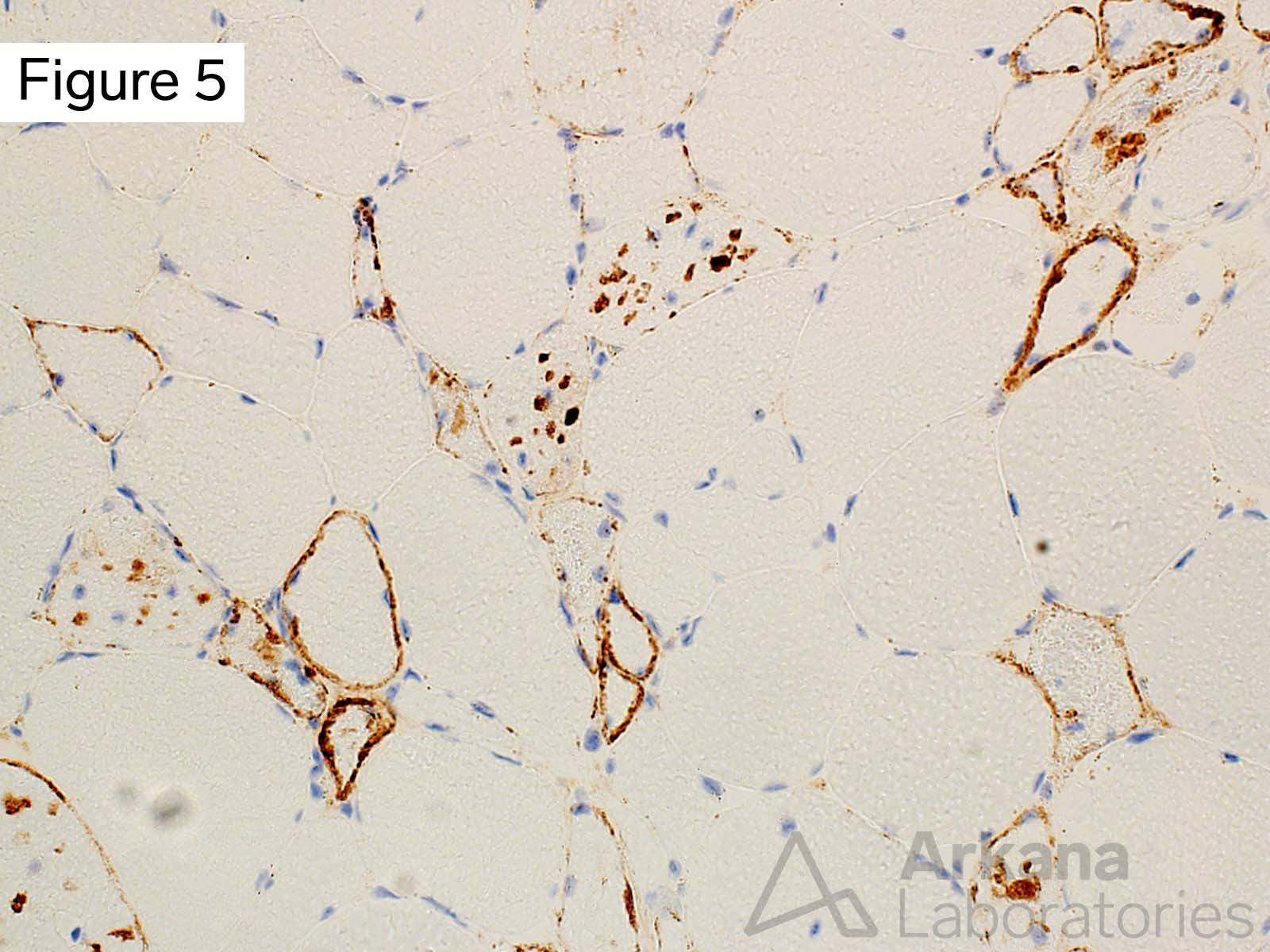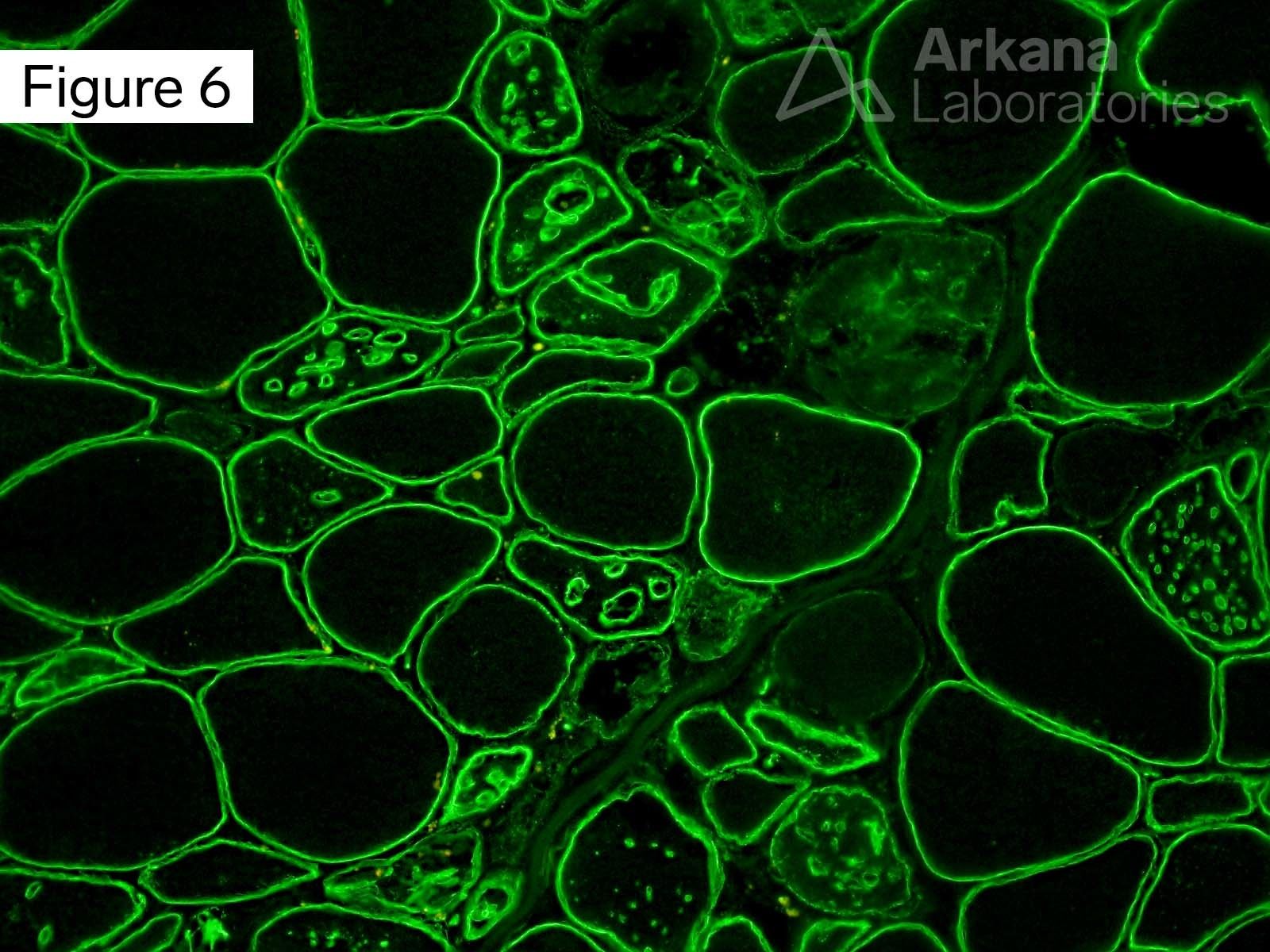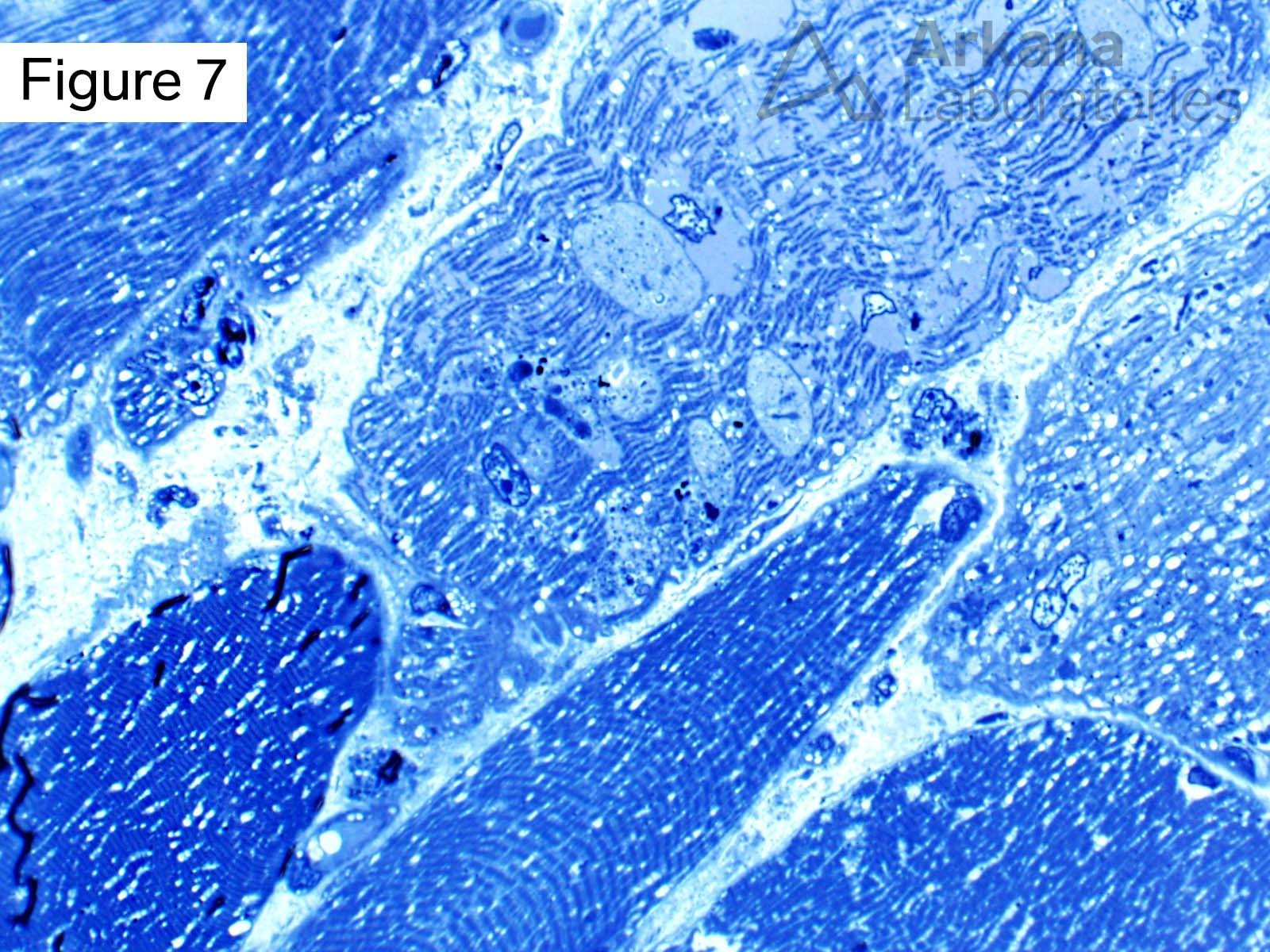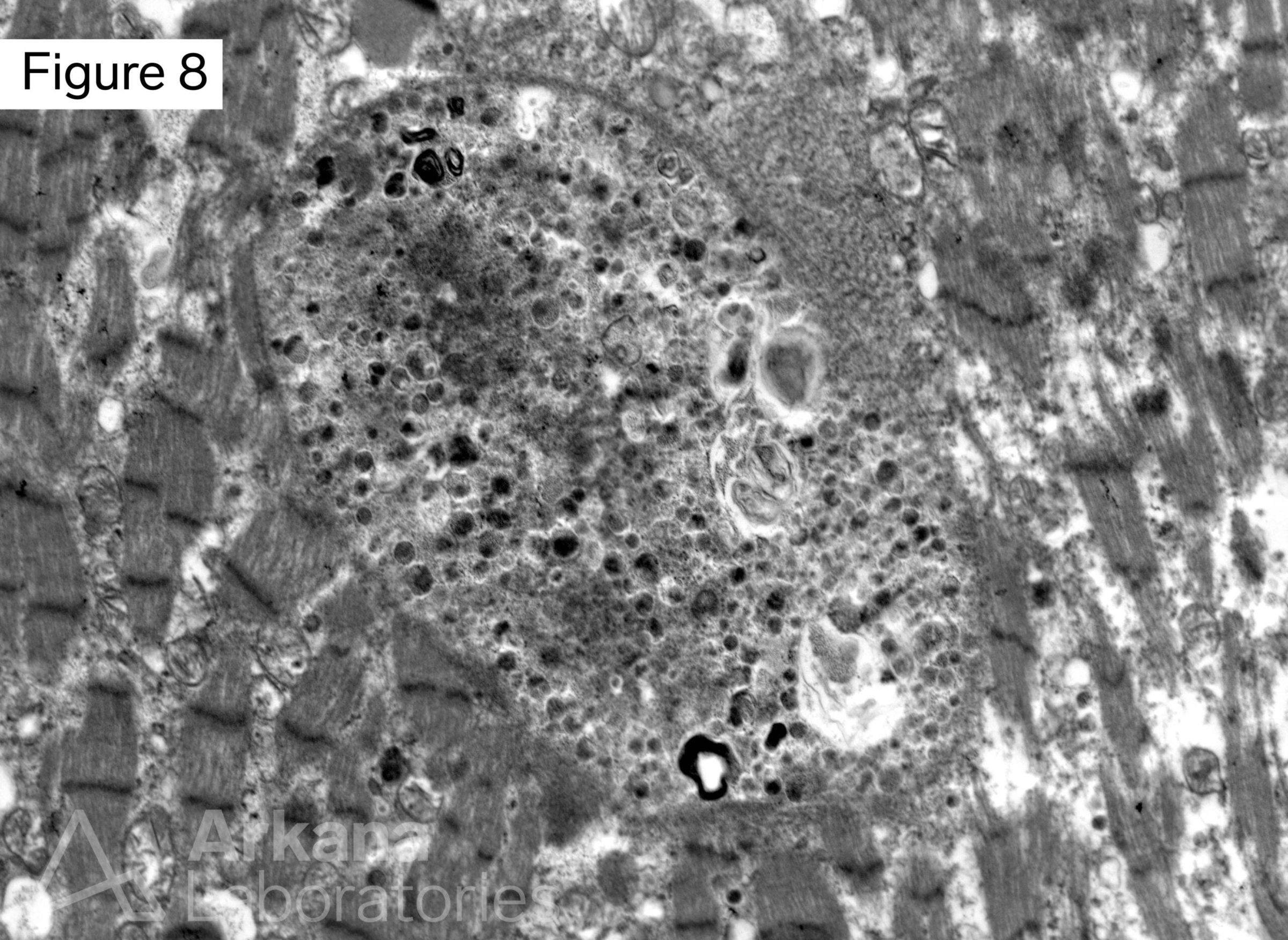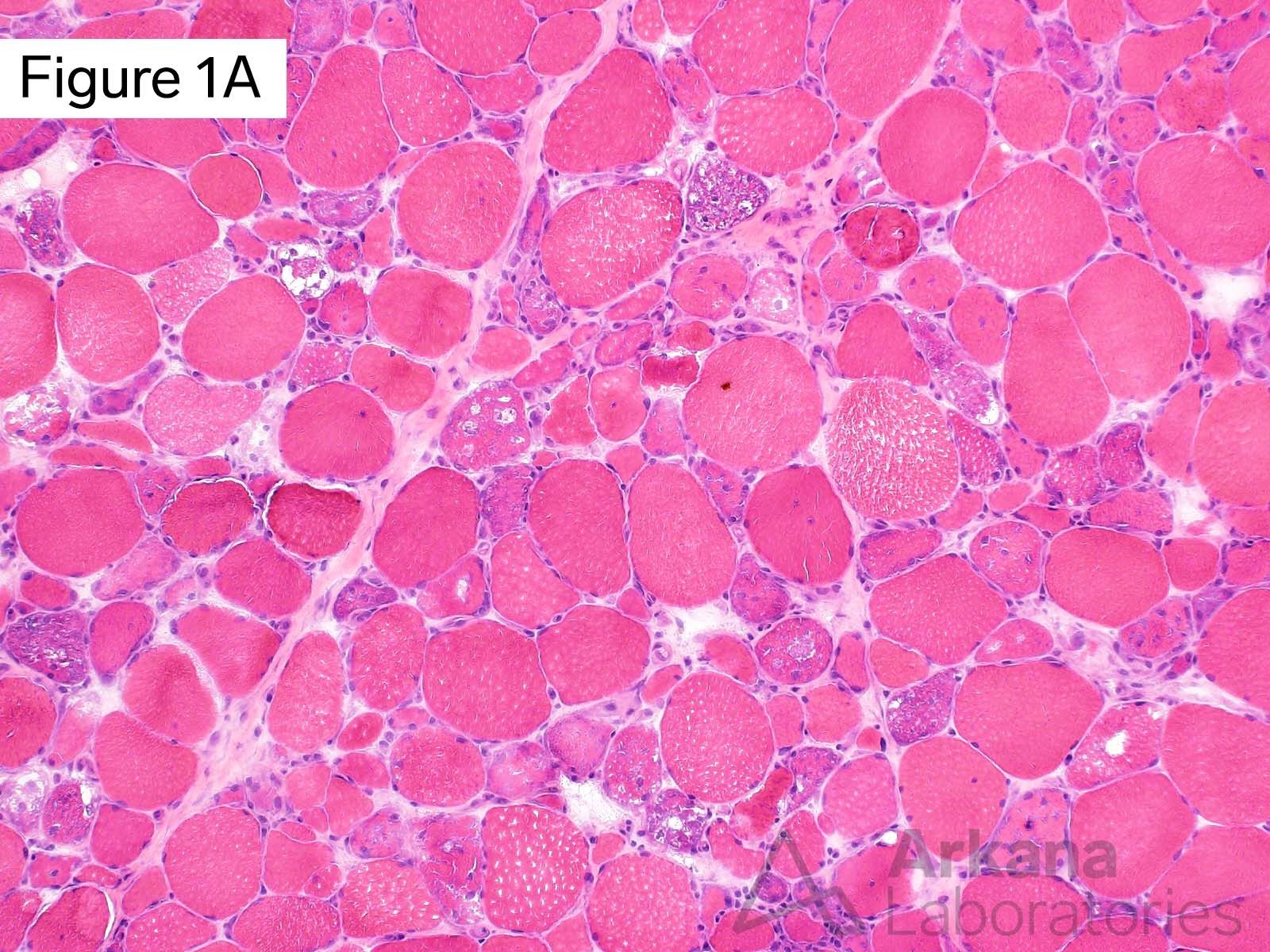
This 50-year-old male presented with profound upper and lower extremity proximal muscle weakness. His family history was negative for neuromuscular disorder. Laboratory studies demonstrated elevated CPK (1150), aldolase and sedimentation rate (26), and normal CRP and TSH. A myositis-specific autoantibody panel was negative. The patient’s home medications did not include a statin, hydroxychloroquine, or colchicine.
Which of the following is the most appropriate pathologic diagnosis based on images #1 through #8?
A. Dermatomyositis
B. Inclusion Body Myositis
C. Statin myopathy
D. Vacuolar myopathy
Answer: Vacuolar myopathy
The morphologic features seen on H&E and modified Gomori Trichrome stained sections fit under the general umbrella term of “vacuolar myopathy” which has multiple potential causes (including Pompe disease, and toxic myopathies related to colchicine or hydroxychloroquine). The staining of the vacuoles for proteins that are normally associated with the sarcolemma (cell membrane of muscle fibers) such as dystrophin, indicates the presence of “Autophagic Vacuoles with Sarcolemmal Features.” The finding of AVSFs narrows the differential diagnosis for this patient’s vacuolar myopathy:
- Danon disease LAMP2 Xq24
- X-linked Myopathy with Excessive Autophagy XMEA VMA21 Xq28
- Desminopathy DES 2q25
- CLN3-related autophagic vacuolar myopathy CLN3 16p12.1
These diseases are examples of disordered autophagy (Greek “self-eating”), an evolutionarily conserved homeostatic process for trafficking cytoplasmic material and organelles for degradation and reuse.
References/Additional Reading
Sugie K, Noguchi S, Kozuka Y, Arikawa-Hirasawa E, Tanaka M, Yan C, Saftig P, von Figura K, Hirano M, Ueno S, Nonaka I, Nishino I. Autophagic vacuoles with sarcolemmal features delineate Danon disease and related myopathies. J Neuropathol Exp Neurol. 2005 Jun;64(6):513-22. doi: 10.1093/jnen/64.6.513. PMID: 15977643.
Rowland TJ, Sweet ME, Mestroni L, Taylor MR. Danon disease – dysregulation of autophagy in a multisystem disorder with cardiomyopathy. J Cell Sci. 2016 Jun 1;129(11):2135-43. doi: 10.1242/jcs.184770. Epub 2016 May 10. PMID: 27165304; PMCID: PMC4920246.
Dowling JJ, Moore SA, Kalimo H, Minassian BA. X-linked myopathy with excessive autophagy: a failure of self-eating. Acta Neuropathol. 2015 Mar;129(3):383-90. doi: 10.1007/s00401-015-1393-4. Epub 2015 Feb 3. PMID: 25644398.
Munteanu I, Ramachandran N, Ruggieri A, Awaya T, Nishino I, Minassian BA. Congenital autophagic vacuolar myopathy is allelic to X-linked myopathy with excessive autophagy. Neurology. 2015 Apr 21;84(16):1714-6. doi: 10.1212/WNL.0000000000001499. Epub 2015 Mar 27. Erratum in: Neurology. 2019 Aug 20;93(8):371. PMID: 25817839; PMCID: PMC4409585.
Weihl CC, Iyadurai S, Baloh RH, Pittman SK, Schmidt RE, Lopate G, Pestronk A, Harms MB. Autophagic vacuolar pathology in desminopathies. Neuromuscul Disord. 2015 Mar;25(3):199-206. doi: 10.1016/j.nmd.2014.12.002. Epub 2014 Dec 12. PMID: 25557463; PMCID: PMC4355324.
Cortese A, Tucci A, Piccolo G, Galimberti CA, Fratta P, Marchioni E, Grampa G, Cereda C, Grieco G, Ricca I, Pittman A, Ciscato P, Napoli L, Lucchini V, Ripolone M, Violano R, Fagiolari G, Mole SE, Hardy J, Moglia A, Moggio M. Novel CLN3 mutation causing autophagic vacuolar myopathy. Neurology. 2014 Jun 10;82(23):2072-6. doi: 10.1212/WNL.0000000000000490. Epub 2014 May 14. PMID: 24827497; PMCID: PMC4118497.
Parzych KR, Klionsky DJ. An overview of autophagy: morphology, mechanism, and regulation. Antioxid Redox Signal. 2014 Jan 20;20(3):460-73. doi: 10.1089/ars.2013.5371. Epub 2013 Aug 2. PMID: 23725295; PMCID: PMC3894687.
Galluzzi L, Baehrecke EH, Ballabio A, Boya P, Bravo-San Pedro JM, Cecconi F, Choi AM, Chu CT, Codogno P, Colombo MI, Cuervo AM, Debnath J, Deretic V, Dikic I, Eskelinen EL, Fimia GM, Fulda S, Gewirtz DA, Green DR, Hansen M, Harper JW, Jäättelä M, Johansen T, Juhasz G, Kimmelman AC, Kraft C, Ktistakis NT, Kumar S, Levine B, Lopez-Otin C, Madeo F, Martens S, Martinez J, Melendez A, Mizushima N, Münz C, Murphy LO, Penninger JM, Piacentini M, Reggiori F, Rubinsztein DC, Ryan KM, Santambrogio L, Scorrano L, Simon AK, Simon HU, Simonsen A, Tavernarakis N, Tooze SA, Yoshimori T, Yuan J, Yue Z, Zhong Q, Kroemer G. Molecular definitions of autophagy and related processes. EMBO J. 2017 Jul 3;36(13):1811-1836. doi: 10.15252/embj.201796697. Epub 2017 Jun 8. PMID: 28596378; PMCID: PMC5494474.
Levine B, Kroemer G. Biological Functions of Autophagy Genes: A Disease Perspective. Cell. 2019 Jan 10;176(1-2):11-42. doi: 10.1016/j.cell.2018.09.048. PMID: 30633901; PMCID: PMC6347410.
Quick note: This post is to be used for informational purposes only and does not constitute medical or health advice. Each person should consult their own doctor with respect to matters referenced. Arkana Laboratories assumes no liability for actions taken in reliance upon the information contained herein.