Welcome to our first post in the Art of Medicine series! In the hustle and bustle of busy clinics, labs, and hospitals we are often focused on the practical aspects of the practice of medicine. Dr. Tiffany Caza is the content creator for this series as well as being a renal pathologist and artist. Her art focuses on her interpretations of the histologic, cellular, and sub-cellular milieu where a dazzling variety of chemical and structural forces inherent to all life exist. In this series, she will be sharing her art (and occasionally others) and providing correlates to the science represented in her interpretations. This series is intended to remind us all that behind our daily practice of medicine, there is another world where the Art of Medicine lives.
And with that, we’ll let Dr. Caza take it away!
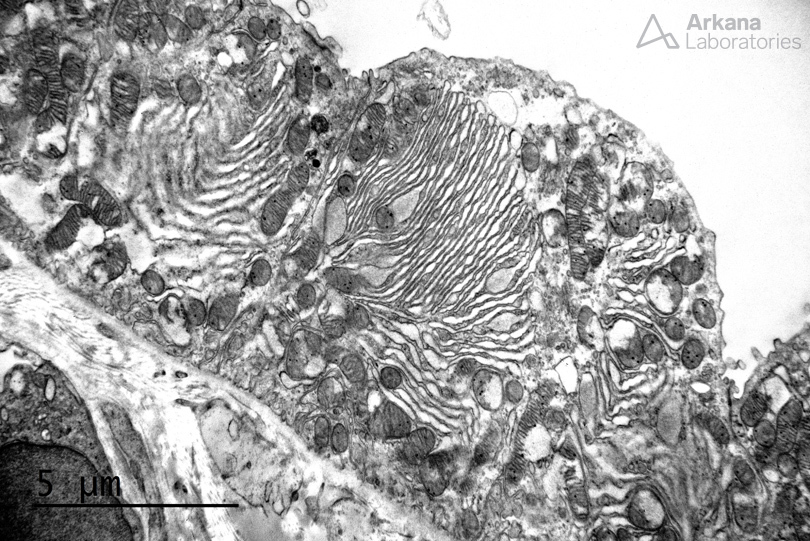
Uromodulin-related kidney disease is an autosomal dominant tubulointerstitial nephropathy resulting from mutations in the UMOD gene on chromosome 16p12 (Nasr et al, 2008). In a recent study of 3315 patients from two large cohorts with chronic kidney disease (CKD), monogenic genetic disorders were identified in 9.3%. Genetic disorders may be an under-recognized source of CKD in adults. Six mutations were found to make up 63% of all genetic diagnoses in CKD patients, of which included UMOD (Groopman et al, 2018). UMOD mutations result in a defect in protein folding, resulting in accumulation of uromodulin within the endoplasmic reticulum that is unable to be secreted. On electron microscopy, increased endoplasmic reticulum and segments of bundled and dilated endoplasmic reticulum are identified within tubular epithelial cells (shown below). Histopathologic features on light microscopy include a disproportionate degree of tubulointerstitial scarring to the degree of glomerular scarring, tubular microcysts in distal nephron segments, and intraepithelial inclusions (which contain dilated and bundled endoplasmic reticulum as shown in both images).
References:
Groopman EE, Marasa M, Cameron-Christie S, Petrovski S, Aggarwal VS, Milo-Rasouly H, Li Y, Zhang J, Nestor J, Krithivasan P, Lam WY, Mitrotti A, Piva S, Kil BH, Chatterjee D, Reingold R, Bradbury D, DiVecchia M, Snyder H, Mu X, Mehl K, Balderes O, Fasel DA, Weng C, Radhakrishnan J, Canetta P, Appel GB, Bomback AS, Ahn W, Uy NS, Alam S, Cohen DJ, Crew RJ, Dube GK, Rao MK, Kamalakaran S, Copeland B, Ren Z, Bridgers J, Malone CD, Mebane CM, Dagaonkar N, Fellstrom BC, Haefliger C, Mohan S, Sanna-Cherchi S, Kiryluk K, Fleckner J, March R, Platt A, Goldstein DB, Gharavi AG. Diagnostic Utility of Exome Sequencing for Kidney Disease. New England Journal of Medicine; 2018 Dec 26, e-pub ahead of print.
https://www.ncbi.nlm.nih.gov/pubmed/30586318
Nasr SH, Lucia JP, Galgano SJ, Markowitz GS, D’Agati VD. Uromodulin storage disease. Kidney Int. 2008 Apr;73(8):971-6.
https://www.ncbi.nlm.nih.gov/pubmed/18004297
Quick note: This post is to be used for informational purposes only and does not constitute medical or health advice. Each person should consult their own doctor with respect to matters referenced. Arkana Laboratories assumes no liability for actions taken in reliance upon the information contained herein.