This biopsy is from a 9-year-old boy who developed pediatric nephrotic syndrome in the first year of life and has been maintained on cyclosporine (CSA) due to frequent relapses. He had not had a biopsy before and was clinically on remission, considering switching to rituximab. The biopsy was performed to assess chronic changes and the presence of histologic signs of chronic calcineurin inhibitor (CNI) toxicity CNI toxicity. His serum creatinine was 0.6 mg/dL. He was also on lisinopril for proteinuria control. He was not hypertensive. The biopsy contained a total of 61 glomeruli, four of which were globally sclerotic. No areas of segmental glomerular sclerosis were seen. A few glomeruli had a fetal appearance (Figure 1). Many glomeruli displayed prominent juxtaglomerular apparata (JGA), (Figure 2). Focal mild arteriolar hyalinosis was also present (Figure 3). Electron microscopy revealed well-preserved foot processes. A diagnosis of focal global glomerulosclerosis with mild chronic changes and features consistent with chronic calcineurin toxicity was made. JGA hyperplasia can be seen in hyperreninemic states such as Bartter and Gitelman Syndrome, as well as renal artery stenosis, among others (https://www.arkanalabs.com/jga-hyperplasia/). These were not present in the current case. Although JGA hyperplasia is not commonly featured as one of the morphologic findings in chronic CNI toxicity (1); in vitro studies have shown that cyclosporine can enhance renin secretion from JGA cells (2) and result in increased levels of TGF-β in vivo (3), which has a mitogenic effect and could potentially mediate the development of JGA hyperplasia on the long-term. An increase in the number of renin-positive cells along arterioles has also been observed in a biopsy series of 26 children with pediatric nephrotic syndrome maintained on moderate dose cyclosporine, in which pre- and post-cyclosporine biopsies were compared (4). This was independent of the underlying degree of interstitial fibrosis and tubular atrophy. In an additional subset of 7 children who underwent repeat biopsy after CSA withdrawal, renin immunoreactivity decreased; further supporting a role for CSA in increasing renin expression in these children. The use of moderate doses of CSA for proteinuric control in childhood nephrotic syndrome, as opposed to relatively lower doses as maintenance immunosuppression in transplant recipients may explain why JGA hyperplasia is not commonly seen in allograft biopsies from patients on CNIs.
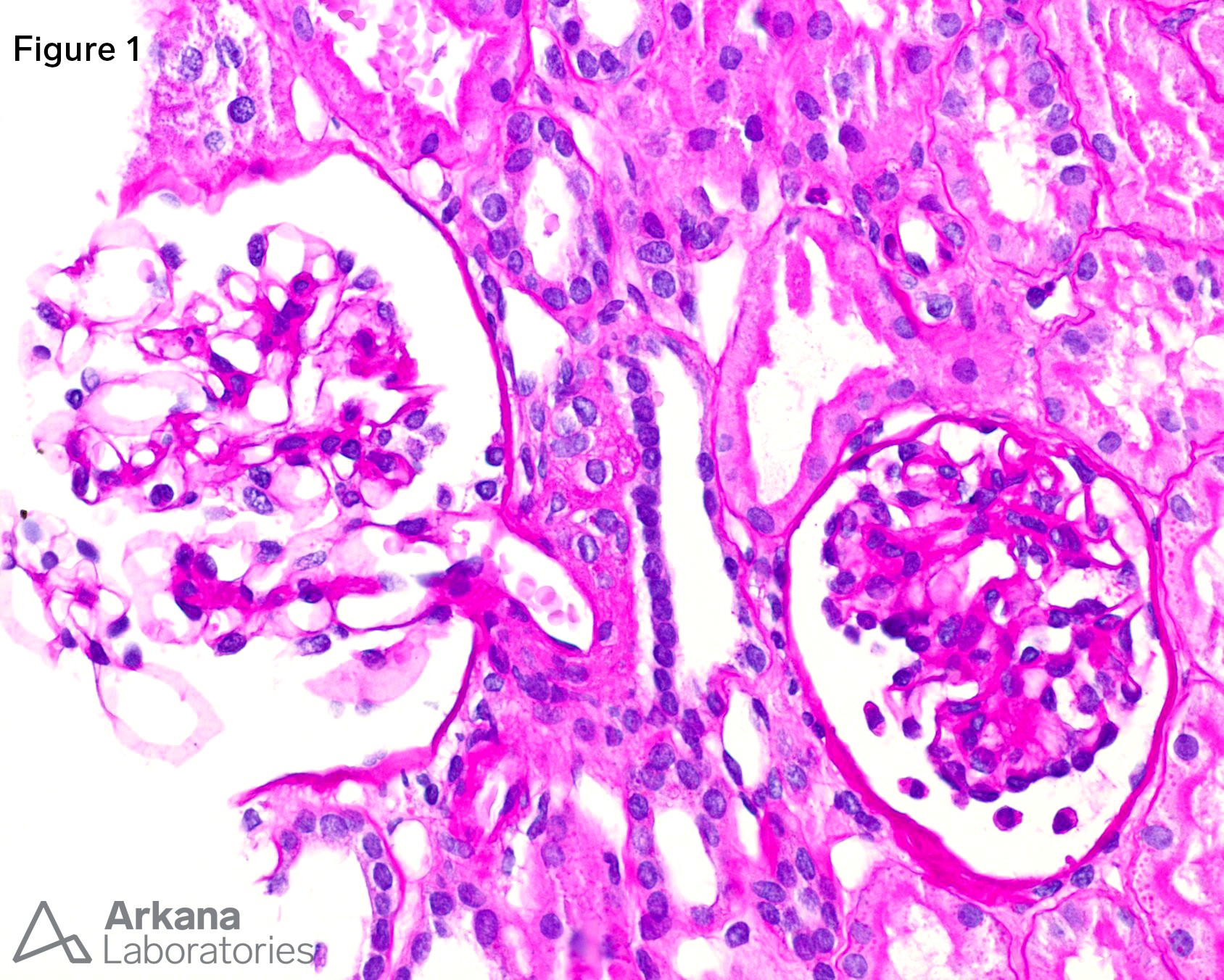
Figure 1. Glomerulus with prominent podocytes and a fetal appearance beside a normal glomerulus
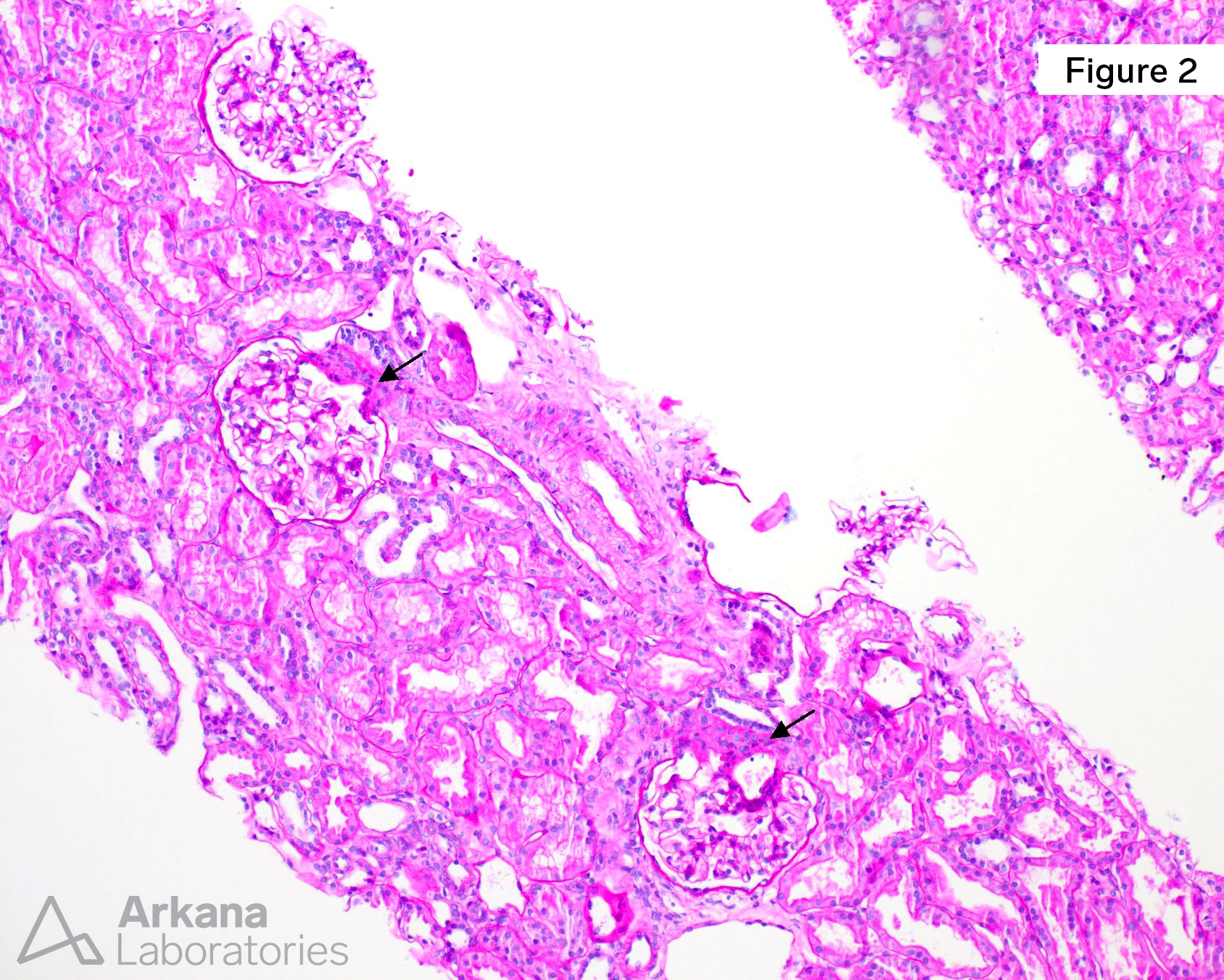
Figure 2. Most glomeruli displayed prominent JGA, readily identifiable at scanning magnification
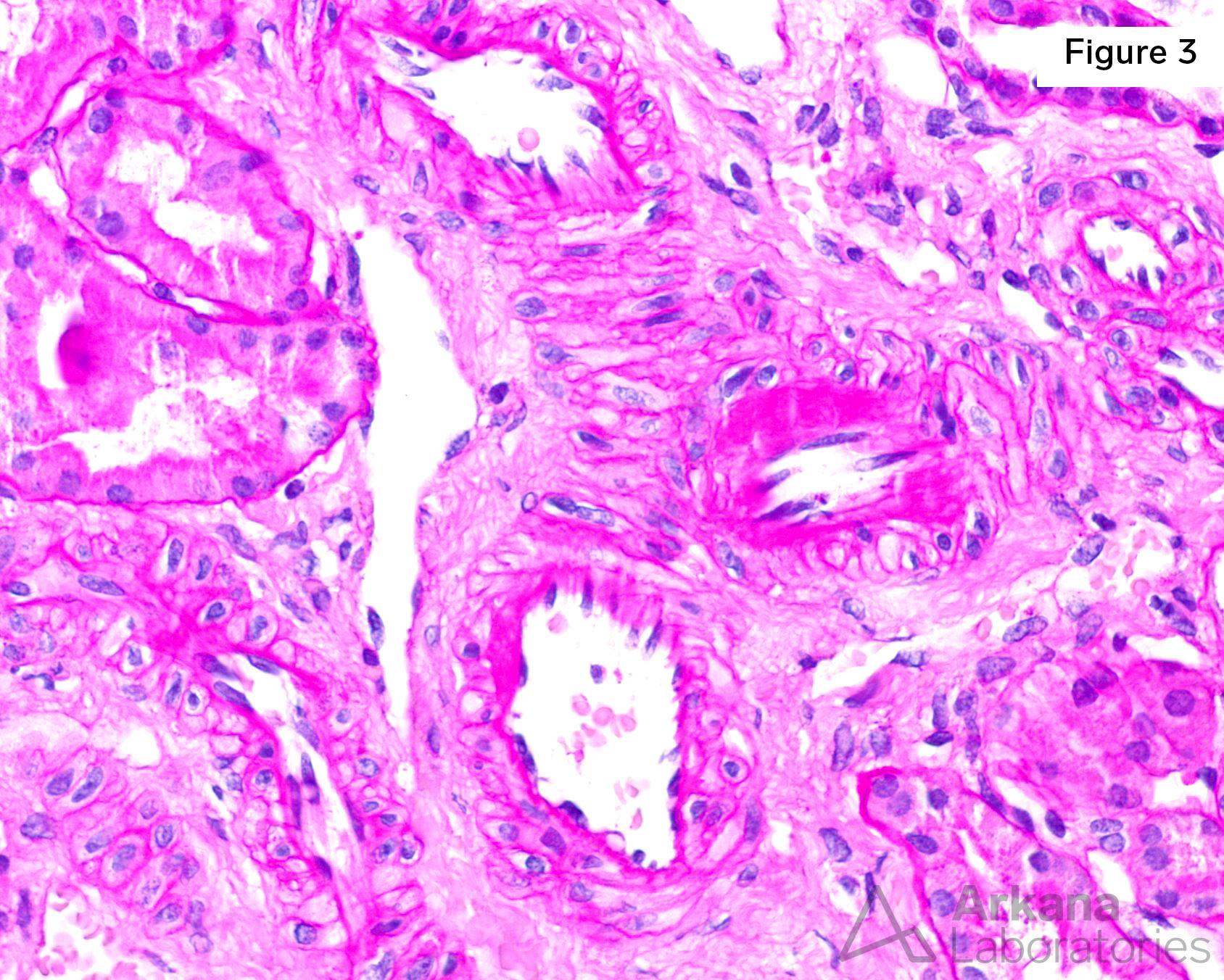
Figure 3. Mild arteriolar hyalinosis in this 9-year-old child with no hypertension or diabetes is likely the result of chronic cyclosporine use
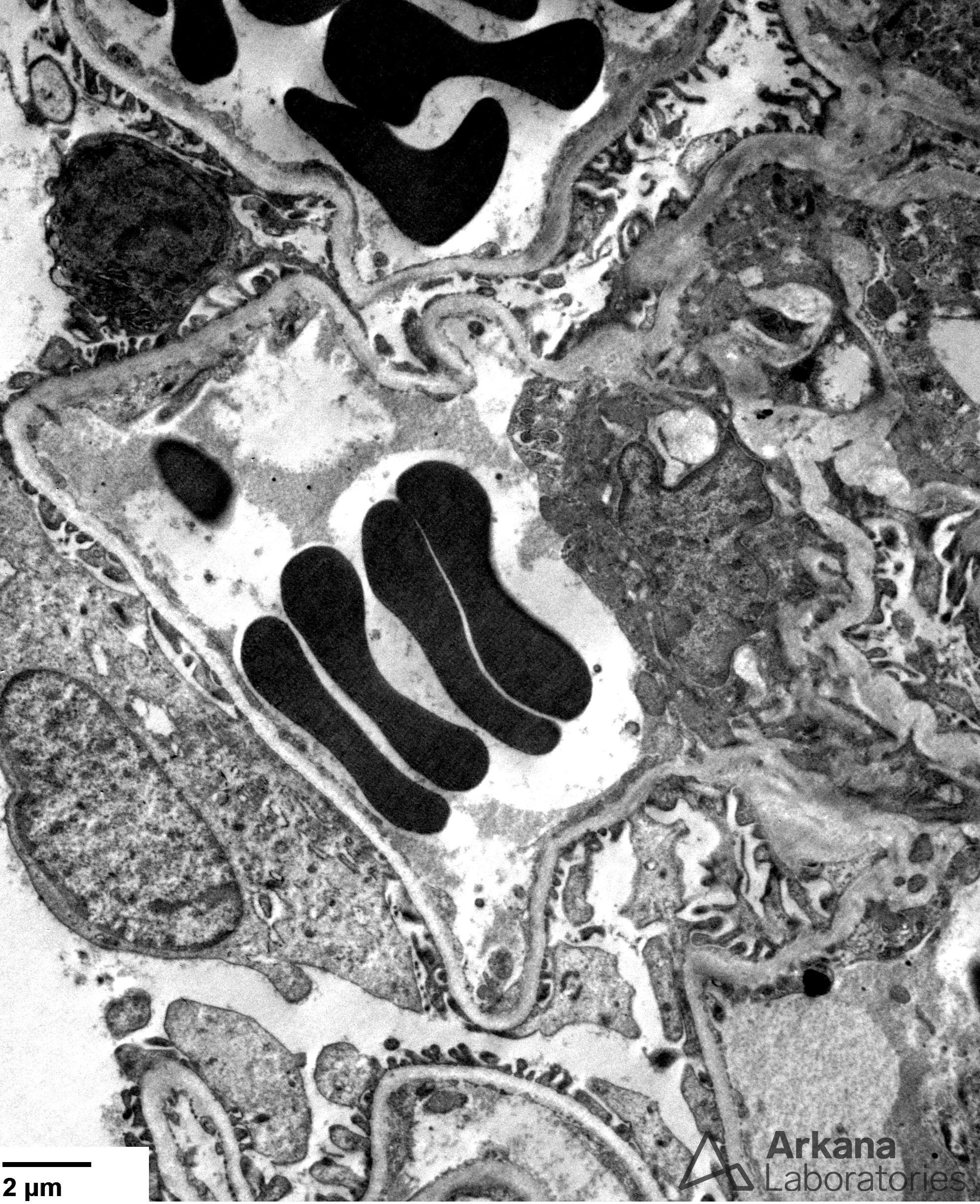
Figure 4. Well-preserved foot processes were apparent on ultrastructural examination, consistent with the fact that this patient was clinically in remission
References:
Naesens M, Kuypers DR, Sarwal M. Calcineurin inhibitor nephrotoxicity. Clinical Journal of the American Society of Nephrology. 2009 Feb 1;4(2):481-508.
Kurtz A, Della Bruna R, Kühn K. Cyclosporine A enhances renin secretion and production in isolated juxtaglomerular cells. Kidney international. 1988 May 1;33(5):947-53.
Shehata M, Cope GH, Johnson TS, Raftery AT, El Nahas AM. Cyclosporine enhances the expression of TGF-β in the juxtaglomerular cells of the rat kidney. Kidney international. 1995 Nov 1;48(5):1487-96.
Iijima K, Hamahira K, Kobayashi A, Nakamura H, Yoshikawa N. Immunohistochemical analysis of renin activity in chronic cyclosporine nephropathy in childhood nephrotic syndrome. Journal of the American Society of Nephrology. 2000 Dec 1;11(12):2265-71.
Quick note: This post is to be used for informational purposes only and does not constitute medical or health advice. Each person should consult their own doctor with respect to matters referenced. Arkana Laboratories assumes no liability for actions taken in reliance upon the information contained herein.