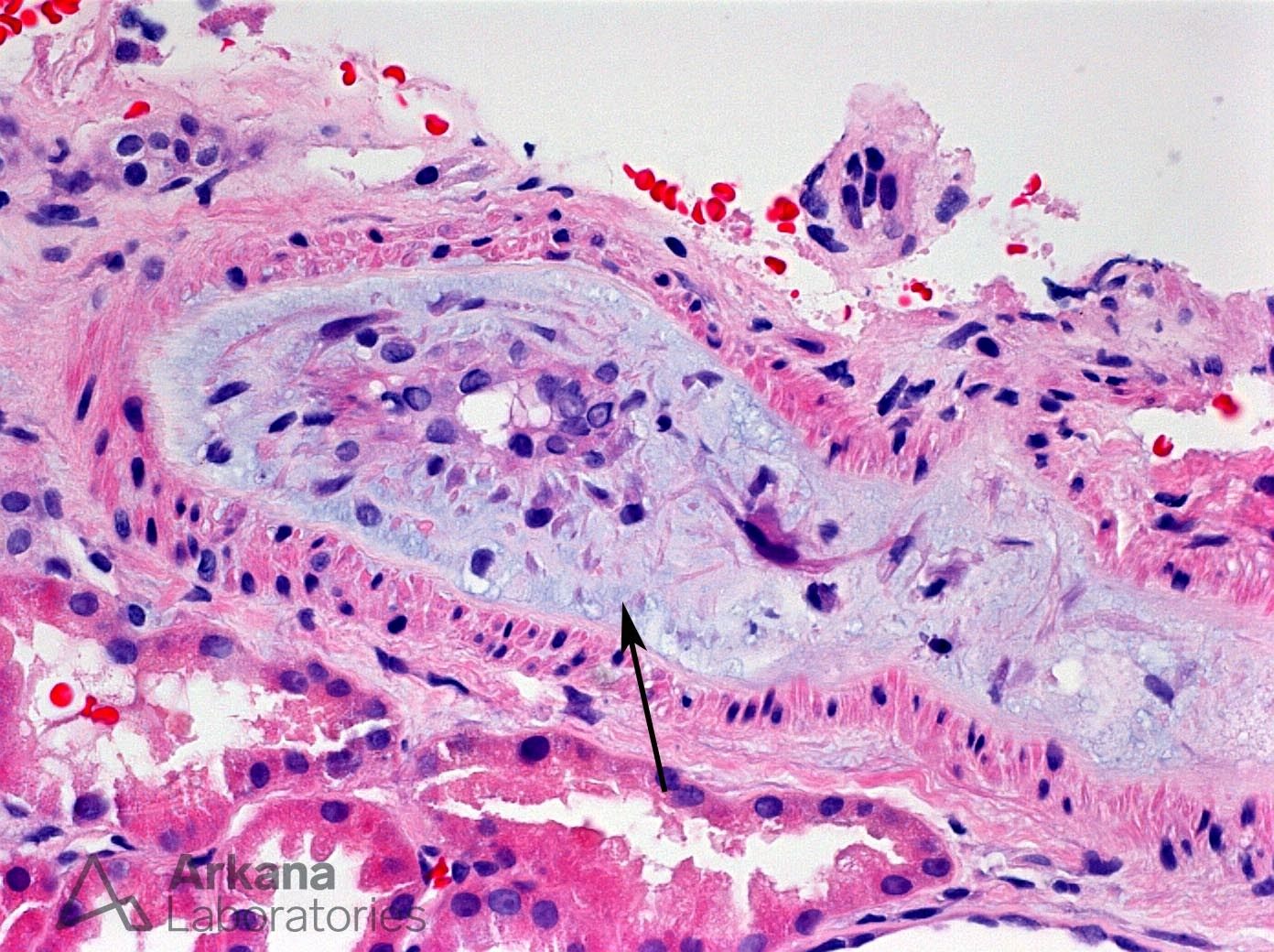
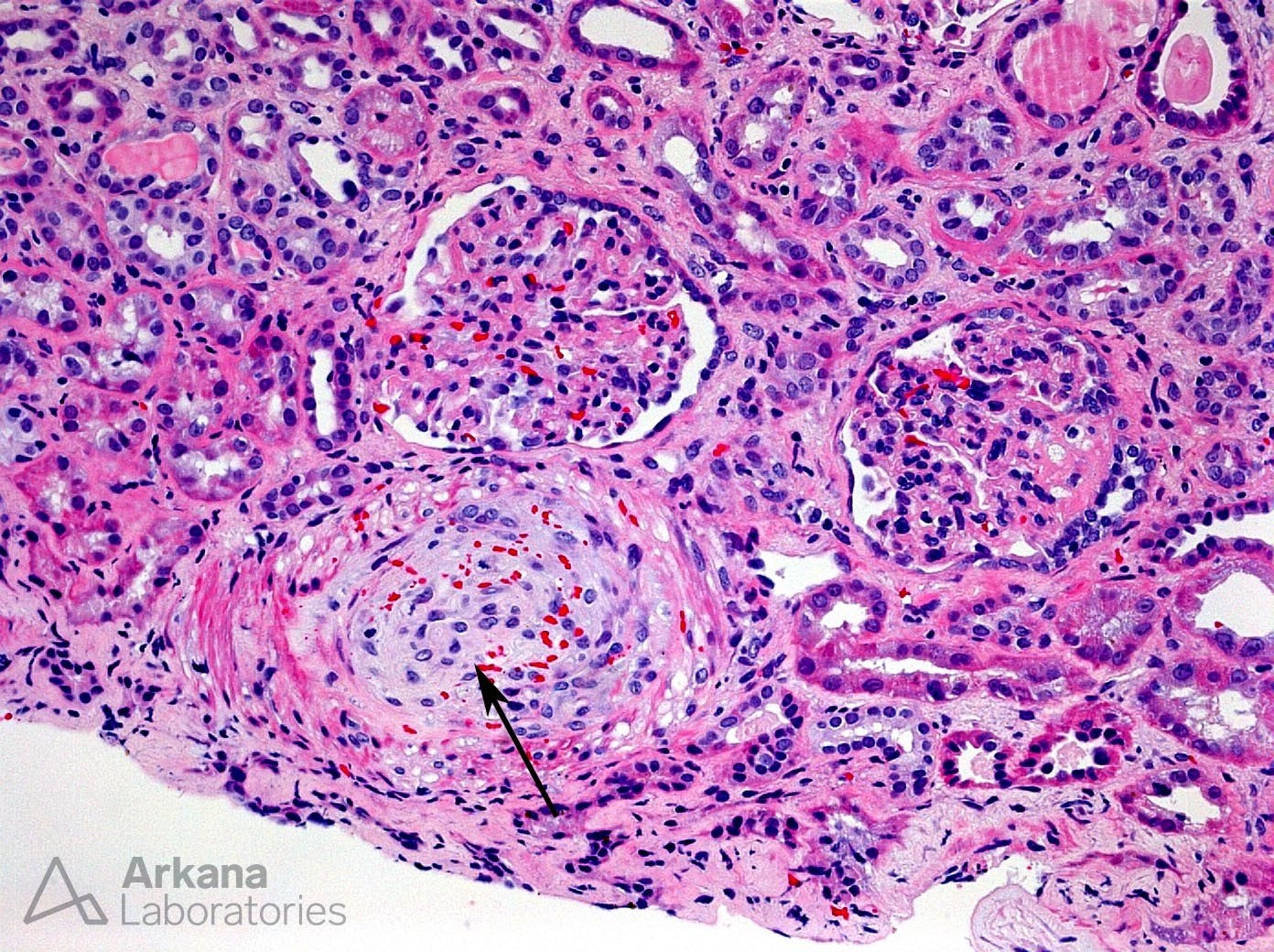
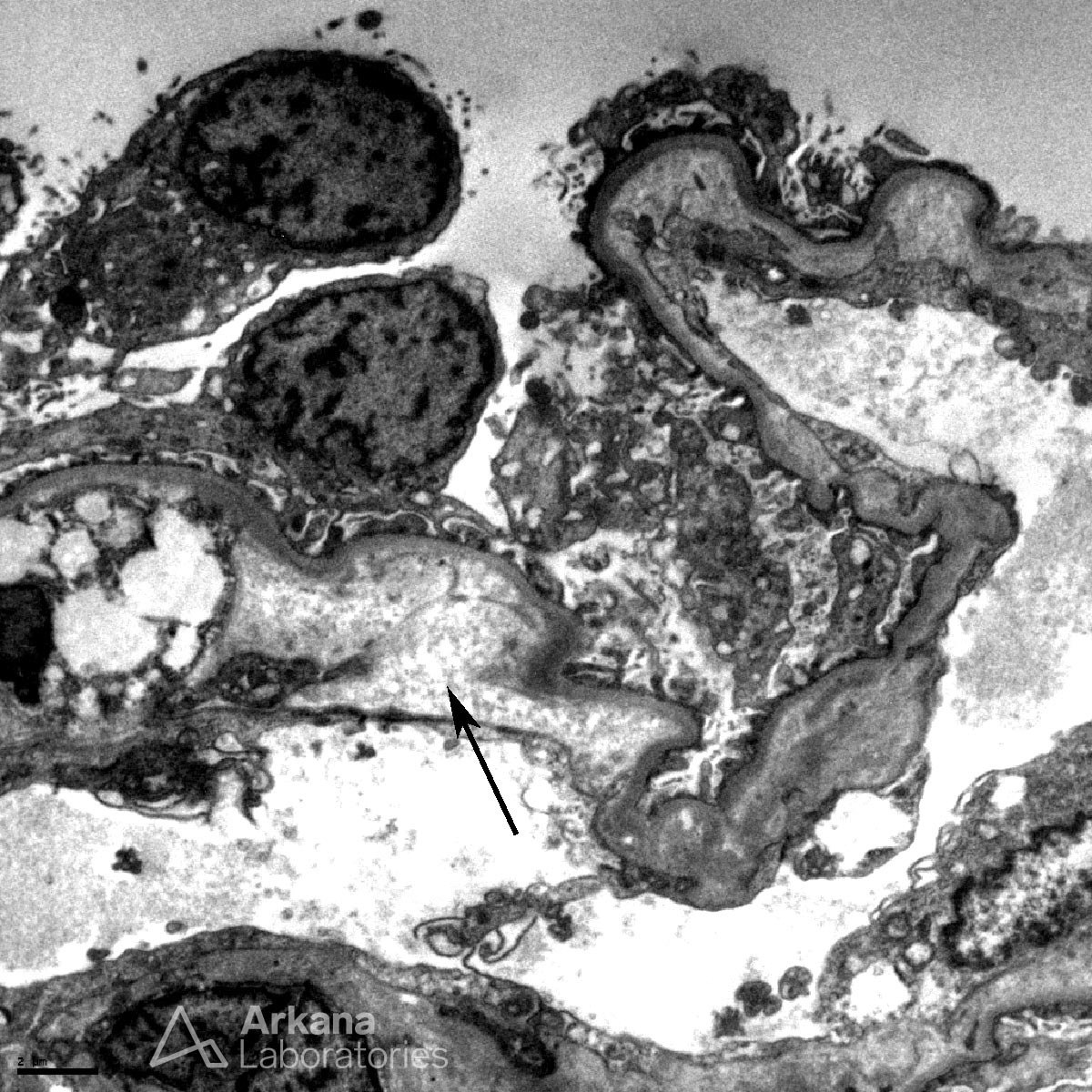
The histologic features of thrombotic microangiopathy (TMA) associated with intravenous abuse of extended-release oxymorphone hydrochloride (Opana ER) are shown in these photomicrographs. Medium-power view of an interlobular-sized artery shows severe mucoid intimal edema and associated fractured RBCs (arrow). Adjacent glomeruli show ischemic basement membrane wrinkling. A high-power view of same biopsy specimen shows near-luminal occlusion of an interlobular-sized artery secondary to severe endothelial cell swelling and mucoid intimal edema. By electron microscopy, glomerular basement membranes showed marked subendothelial electron-lucent expansion (arrow).
Oral extended-release oxymorphone hydrochloride (Opana ER) is an opioid agonist that has undergone a tamper-resistant reformulation. A report by the CDC included 15 patients from Tennessee who presented with a TTP-like illness associated with injection of this reformulated drug.1 Kidney biopsies from patients with Opana-associated TTP-like illness show the changes demonstrated in the photomicrographs presented here, with severe arterial mucoid intimal edema and resultant glomerular ischemia.2 Kidney outcome is poor in most cases.
References:
1. Centers for Disease Control and Prevention (CDC). Thrombotic thrombocytopenic purpura (TTP)–like illness associated with intravenous opana ER abuse — Tennessee, 2012. MMWR Morb Mortal Wkly Rep. 2013; 62: 1-16.
2. Ambruzs JM, Serrell PB, Rahim N, et al. Thrombotic microangiopathy and acute kidney injury associated with intravenous abuse of an oral extended-release formulation of oxymorphone hydrochloride: kidney biopsy findings and report of 3 cases. Am J Kidney Dis 2014; 63 1022-1026
Quick note: This post is to be used for informational purposes only and does not constitute medical or health advice. Each person should consult their own doctor with respect to matters referenced. Arkana Laboratories assumes no liability for actions taken in reliance upon the information contained herein.