Monday:
Welcome to ANCA-associated Vasculitis (AAV) Disease Week! We will walk you through the history, pathogenesis, pathological features, disease classification, prognosis, and recent advances in the management of ANCA-associated vasculitis during this week.

The first few cases of necrotizing and crescentic GN associated with the presence of a serum factor that stained neutrophils cytoplasm were described in Australia in 1982. Due to case clustering and positive serologies for the Ross River virus, these cases were initially suspected to be related to this arbovirus.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1499415/

Subsequently, the pathogenic role of anti-neutrophil cytoplasmic antibodies in the disease was elucidated by Jennette and Falk in 1988.
https://pubmed.ncbi.nlm.nih.gov/2453802/
The two major target antigens of ANCA antibodies are MPO and PR3 in neutrophils granules and lysosomes of monocytes. Another recently described auto-antigen is the lysosomal-associated membrane protein 2 (LAMP2).
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3935581/
In 10-15% of patients with pauci-immune focal necrotizing crescentic GN, ANCAs cannot be detected. Some of these patients may harbor LAMP2 antibodies while others may have anti-pentraxin 3 antibodies.
https://pubmed.ncbi.nlm.nih.gov/26797217/
Evidence for ANCAs being not just a serologic marker but active players in the pathogenesis of vasculitis comes from in vitro studies as well as animal models.
https://doi.org/10.1172/JCI15918.
Which of the following is evidence that ANCAs are pathogenic?
a) Incubation of ANCAs with primed neutrophils leads to the release of mediators that can injure endothelial cells.
b) The infusion of anti-MPO IgG in mice leads to focal necrotizing and crescentic GN with no immunocomplex deposition (pauci-immune).
c) MPO knockout mice develop crescentic GN when immunized with mice MPO.
d) Mice lacking functioning B and T lymphocytes develop crescentic GN when injected with splenocytes from MPO immunized mice.
e) All of the above
Answer: E- all of the above
Further support for the pathogenic role of ANCA in the development of vasculitis comes from a report of neonatal pulmonary-renal syndrome occurring after transplacental passage of MPO-ANCA antibodies.
https://pubmed.ncbi.nlm.nih.gov/15521377/
No convincing animal model of PR3-ANCA vasculitis exists; however, a genome-wide association study did identify PR3 SNPs as well as SERPINA1 (the gene encoding α1-antitrypsin, of which PR3 is a substrate) in association with PR3-ANCA vasculitis.
Patients with α1-antitrypsin deficiency may have increased susceptibility to the development of AAV.
https://doi.org/10.1111/j.1365-2796.1994.tb00842.x
PR3-AAV was also associated with HLA-DP; MPO-AAV was only associated with SNPS in HLA-DQ region. These HLA associations may also underly the different geographic distribution of ANCA vasculitis, with GPA and PR3-ANCA being more common in white European populations and MPA with MPO-ANCAs in Asians.
Importantly, this GWAS also showed these genetic distinctions were associated with ANCA specificity rather than the clinical syndrome, supporting the idea of classifying these diseases according to autoantibody rather than phenotype.
https://www.nejm.org/doi/full/10.1056/nejmoa1108735
Tuesday:
A connection between ANCA vasculitis and infection dates back to the first report of GPA in 1936 when it was described as a granulomatous ‘septic’ vasculitis. And subsequently when the association with ANCAs was made, in a case series thought to be secondary to Ross River virus infection.
One of the mechanisms through which infections may trigger the development of ANCAs is through molecular mimicry and autoantigen complementarity.
Several pathogens have antigens that are homologous to anti-sense PR3 (cPR3), including Staphylococcus aureus, Entamoeba histolytica, and Ross River virus.
https://doi.org/10.1038/ki.2010.472
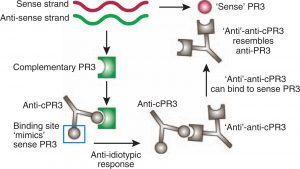
Antibodies against these cPR3 peptides may then induce the development of anti-PR3. Indeed, many patients with ANCA are found to have both anti-PR3 and anti-cPR3 antibodies.
Similarly, the LAMP2 epitope that is recognized by ANCA is homologous to the bacterial adhesion molecular FimH, found in many Gram-negative bacteria such as Escherichia coli, Klebsiella pneumonia, and Proteus mirabilis.
Rats immunized with FimH produced antibodies to rat and human LAMP2 and developed pauci-immune NCGN.
https://pubmed.ncbi.nlm.nih.gov/18836458/
A similar mechanism may also explain the observation of concomitant ANCA and anti-GBM disease in up to 30% of patients.
MPO has significant structural overlap with peroxidasin, a key enzyme in the crosslinking of collagen IV NC1 domain which harbors the anti-GBM cryptic epitope. When anti-peroxidasin antibodies develop, defective NC1 crosslinking may lead to exposure of the cryptic epitope leading to the production of anti-GBM antibodies.
https://doi.org/10.1681/ASN.2018050519
Infectious organisms that have been associated with the development of ANCA vasculitis include:
a) Ross River virus
b) Staphylococcus aureus
c) Entamoaeba hystolytica
d) Gram-negatives such as Escherichia coli, Klebsiella pneumonia, and Proteus mirabilis
e) All of the above
Answer: E- all of the above. The association with infections may also explain the seasonal presentation of ANCA vasculitis, with peaks in winter months. https://pubmed.ncbi.nlm.nih.gov/10095799/
However, not all ANCAs are pathogenic; in fact, many healthy controls are found to have autoantibodies against MPO and PR3.
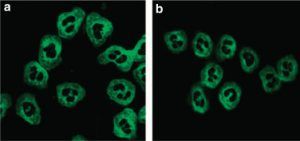
legend: a – perinuclear staining (pANCA, usually anti-MPO); b – cytoplasmic staining (C-ANCA, usually anti-PR3) https://www.sciencedirect.com/science/article/pii/S0085253815545845
This may be because ANCAs targeting different epitopes have different pathogenicity, and/or that a secondary event leading to neutrophil activation is required for the disease to manifest.
ANCA autoantibodies only recognize MPO, PR-3, or LAMP-2:
A. True
B. False
Answer: False. In addition to MPO and PR3, several other neutrophil-derived molecules can be targeted by ANCAs, including α-enolase, azurocidin, bactericidal permeability-increasing protein (BPI), cathepsin G, elastase, defensin, lactoferrin, and moesin.
Most of these atypical ANCAs are not associated with specific diseases, except for ANCAs against elastase, which is associated with cocaine-induced midline destructive lesions.
In resting neutrophils, there is very little to no expression of ANCA antigen targets. Upon neutrophil activation. degranulation and apoptosis; however, these antigens become accessible to ANCA binding.
PR3 and MPO are also present in neutrophil extracellular traps (NETs) that form during neutrophil activation. Defective degradation of NETs could contribute to ANCA formation by prolonging exposure to these antigens.
The anti-thyroid drug, propylthiouracil (PTU) induces a change in MPO that results in abnormal NETs that are resistant to degradation. This may contribute to the breakdown of tolerance to MPO and result in autoantibody production. Indeed, ~30% of patients treated with PTU develop ANCAs.
https://pubmed.ncbi.nlm.nih.gov/12201217/
Other drugs that are associated with ANCA include hydralazine, penicillamine, allopurinol, and sulfasalazine, TNF-a inhibitors, among others.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4491282/
After ANCAs are formed, their binding to MPO or PR3 in primed neutrophils leads to excessive activation, with abnormal cytokine production, the release of ROS and lytic enzymes, as well as further NET formation, all of which injure endothelial cells.
Wednesday:
Today we will go over the pathologic features of ANCA-associated vasculitis in renal biopsies.
Lesions of ANCA-associated vasculitis begin with neutrophilic infiltration, leading to fibrinoid necrosis. Mononuclear cells subsequently surround and replace the neutrophils, leading to granuloma formation.
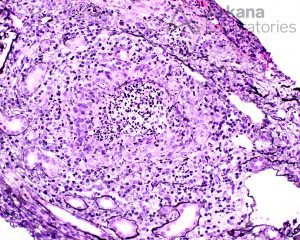
Rarely, interstitial necrotizing lesions and granulomas, not associated with glomeruli or vessels may be seen in renal biopsy.
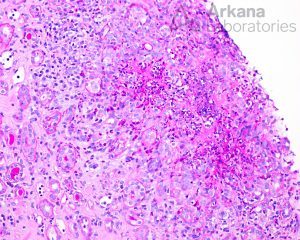
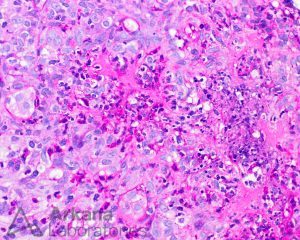
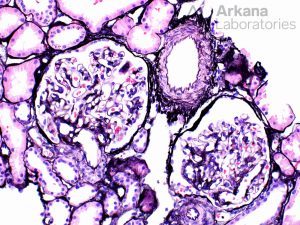
Within glomeruli, the typical lesions are GBM rupture, segmental fibrinoid necrosis, and crescent formation.
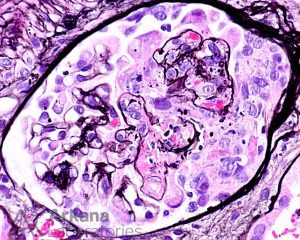
ANCA vasculitis is characterized by repeated bouts of acute lesions, leading to the characteristic finding of both cellular, fibrous-cellular and fibrous crescents within the same biopsy.
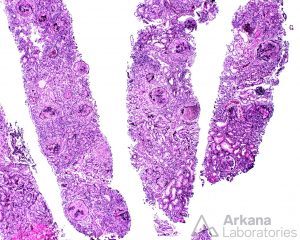
Typically, uninvolved glomeruli show no endocapillary hypercellularity, and there is none to very little immune-complex deposition by immunofluorescence. include the total percentage of global glomerulosclerosis, fibrous crescents, tubular atrophy, and interstitial fibrosis.
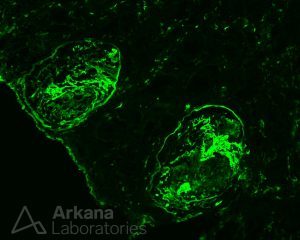
On immunofluorescence, fibrinogen highlights cellular crescents and areas of segmental fibrinoid necrosis.
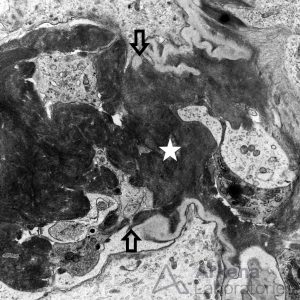
Electron microscopy shows no immune-type deposits, but may show glomerular basement membrane rupture (arrows), in association with fibrin tactoids (star).
Basement Membrane Damage in Crescentic GN
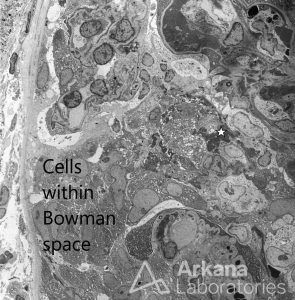
It may also be possible to detect increased numbers of cells within the Bowman’s space in association with extravasation of fibrin (star). There may be extensive foot process effacement due to capillary wall injury; this should not be interpreted as a “podocytopathy”.
Glomerulus With Fibrocellular Crescent
Necrotizing arteritis may also be seen in 10-20% of cases and increases the likelihood of extrarenal (systemic) vasculitis.
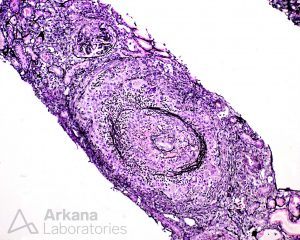
Arteritis is often focal; thus, this incidence is likely an underestimate. A recent study showed that renal arteritis was associated with lower renal survival. JASN 32: 2362–2374, 2021. DOI: https://doi.org/10.1681/ASN.2020071074
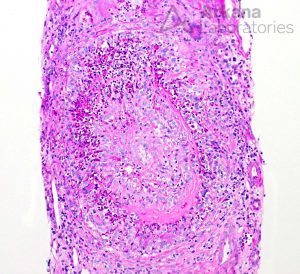
The renal vasa recta capillaries may also be involved. In biopsies with little cortex, the presence of medullary angiitis may support a diagnosis of small vessel vasculitis. However, it may also be seen in other entities tubulointerstitial nephritis, antibiotic use in the setting of infection, IgA nephropathy, and cryoglobulinemic glomerulonephritis, among others.
https://www.sciencedirect.com/science/article/pii/S004681771200233X
If extensive, medullary angiitis may lead to papillary necrosis.
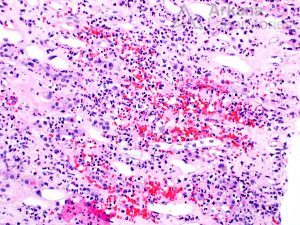
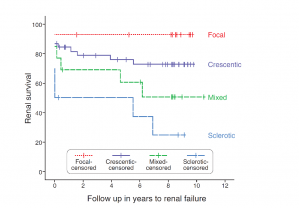
The renal prognosis of patients with ANCA vasculitis is mostly dependent on the proportion of uninvolved (i.e., normal) glomeruli, and extent of scarring, which form the basis of the pathologic classification of ANCA-associated glomerulonephritis.
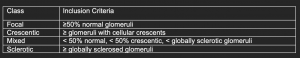
These classes correlated with renal survival in initial studies J Am Soc Nephrol 21: 1628–1636, 2010. DOI: 10.1681/ASN.2010050477. However, subsequent studies have shown significant overlap between crescentic and mixed classes in terms of renal survival.
https://doi.org/10.2215/CJN.14561119
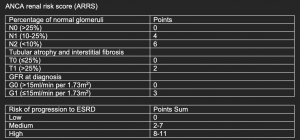
In an attempt to refine prognostic prediction in AAV, a risk score incorporating histological features (% of normal glomeruli, % of tubulointerstitial scarring) and eGFR at diagnosis was proposed.
ANCA renal risk score (ARRS)
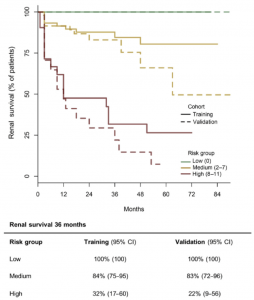
The proposed score accurately predicted ESRD at 36 months in the training cohort and an independent validation cohort. https://doi.org/10.1016/j.kint.2018.07.020
Thursday:
The renal fellow network website summarizes beautifully major randomized clinical trials designed to study treatment strategies for the management of ANCA-associated vasculitis. It always amazes me how amazing the RFN website is as a resource for fellows (and non-fellows as well).
The timeline includes major clinical trials published between 1990 and 2014. Interestingly, since 2014, we had the publication of major trials that have had a tremendous impact on the management of those affected by AAV including the PEXIVAS trial and the ADVOCATE trial.
We have seen major paradigm shifts in the management of ANCA-associated vasculitis with the publication of 2 RCTs. In 2020, we saw the publication of the PEXIVAS trial which showed that a regimen with fewer steroids was non-inferior to a standard-dose regimen in relation to death and ESKD. The primary outcome occurred in 92 patients (27.9%) in the reduced-steroid group and in 83 of 325 (25.5%) in the standard-dose group.
https://www.nejm.org/doi/full/10.1056/nejmoa1803537
Serious infections at 1 year were less common in the reduced-dose group (96 patients or 27.2%) than in the standard-dose group (116 patients or 33%). The most interesting thing was to learn that a well-designed RCT examining the dose of steroids had never been conducted up to the PEXIVAS trial.
https://www.nejm.org/doi/full/10.1056/nejmoa1803537

The PEXIVAS trial also demonstrated no benefits in relation to death from any cause or ESKD for patients receiving PLEX. The primary outcome occurred in 100 patients (28.4%) in the PLEX group and in 109 patients (31.0%) in the control group (HR 0.86, 95% CI 0.65 to 1.13, P=0.27).
https://www.nejm.org/doi/full/10.1056/nejmoa1803537
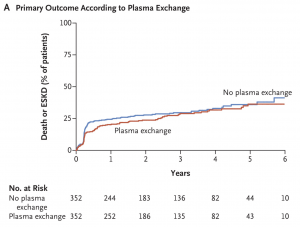
The role of PLEX is still not clear since the results of the PEXIVAS trial conflict with the MEPEX trial which suggested that patients with AAV receiving PLEX could have increased hazard of recovery of kidney function and dialysis independence. The debate is not settled yet about the role of PLEX.
https://jasn.asnjournals.org/content/18/7/2180#ref-15
https://kidney360.asnjournals.org/content/2/5/776
https://kidney360.asnjournals.org/content/2/5/779
In 2021, the ADVOCATE trial was published suggesting that we could move from a glucocorticoid-tapering regimen to one based on inhibition of the complement system with the C5a receptor inhibitor avacopan. Steroids have been the mainstay of treatment of patients with AAV, so it is amazing to think about a steroid-free regimen!
https://www.nejm.org/doi/full/10.1056/nejmoa2023386
Sustained remission at week 52 was detected in 109 patients (65.7%) in the avacopan group and in 90 patients (54.9%) receiving prednisone (P=0.007 for superiority).
https://www.nejm.org/doi/full/10.1056/nejmoa2023386
The ADVOCATE trial was the end result of accumulating evidence demonstrating an important role for the complement system in the pathogenesis of ANCA-associated vasculitis. C5a causes priming of neutrophils by the migration of antigens such as myeloperoxidase (MPO) or proteinase‑3 (PR3) from the cytoplasm to the cell surface. Then, becoming targets for circulating ANCA antibodies.
https://www.nature.com/articles/nrneph.2017.37
Friday:
HISTORY AND PATHOGENESIS OF ANCA-ASSOCIATED VASCULITIS:
The first few cases of necrotizing and crescentic GN associated with the presence of a serum factor that stained neutrophils cytoplasm were described in Australia in 1982. Due to case clustering and positive serologies for the Ross River virus, these cases were initially suspected to be related to this arbovirus. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1499415/
Subsequently, the pathogenic role of anti-neutrophil cytoplasmic antibodies in the disease was elucidated by Jennette and Falk in 1988.
https://pubmed.ncbi.nlm.nih.gov/2453802/
The two major target antigens of ANCA antibodies are MPO and PR3 in neutrophils granules and lysosomes of monocytes. Another recently described auto-antigen is the lysosomal-associated membrane protein 2 (LAMP2).
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3935581/
In 10-15% of patients with pauci-immune focal necrotizing crescentic GN, ANCAs cannot be detected. Some of these patients may harbor LAMP2 antibodies while others may have anti-pentraxin 3 antibodies.
https://pubmed.ncbi.nlm.nih.gov/26797217/
Evidence for ANCAs being not just a serologic marker but active players in the pathogenesis of vasculitis comes from in vitro studies as well as animal models.
https://doi.org/10.1172/JCI15918
Further support for the pathogenic role of ANCA in the development of vasculitis comes from a report of neonatal pulmonary-renal syndrome occurring after transplacental passage of MPO-ANCA antibodies.
https://pubmed.ncbi.nlm.nih.gov/15521377/
No convincing animal model of PR3-ANCA vasculitis exists; however, a genome-wide association study did identify PR3 SNPs as well as SERPINA1 (the gene encoding α1-antitrypsin, of which PR3 is a substrate) in association with PR3-ANCA vasculitis.
Patients with α1-antitrypsin deficiency may have increased susceptibility to the development of AAV.
https://doi.org/10.1111/j.1365-2796.1994.tb00842.x
PR3-AAV was also associated with HLA-DP; MPO-AAV was only associated with SNPS in HLA-DQ region. These HLA associations may also underly the different geographic distribution of ANCA vasculitis, with GPA and PR3-ANCA being more common in white European populations and MPA with MPO-ANCAs in Asians.
Importantly, this GWAS also showed these genetic distinctions were associated with ANCA specificity rather than the clinical syndrome, supporting the idea of classifying these diseases according to autoantibody rather than phenotype.
https://www.nejm.org/doi/full/10.1056/nejmoa1108735
A connection between ANCA vasculitis and infection dates back to the first report of GPA in 1936 when it was described as a granulomatous ‘septic’ vasculitis. And subsequently when the association with ANCAs was made, in a case series thought to be secondary to Ross River virus infection.
One of the mechanisms through which infections may trigger the development of ANCAs is through molecular mimicry and autoantigen complementarity.
Several pathogens have antigens that are homologous to anti-sense PR3 (cPR3), including Staphylococcus aureus, Entamoeba histolytica, and Ross River virus.
https://doi.org/10.1038/ki.2010.472
Antibodies against these cPR3 peptides may then induce the development of anti-PR3. Indeed, many patients with ANCA are found to have both anti-PR3 and anti-cPR3 antibodies.
Similarly, the LAMP2 epitope that is recognized by ANCA is homologous to the bacterial adhesion molecular FimH, found in many Gram-negative bacteria such as Escherichia coli, Klebsiella pneumonia, and Proteus mirabilis.
Rats immunized with FimH produced antibodies to rat and human LAMP2 and developed pauci-immune NCGN.
https://pubmed.ncbi.nlm.nih.gov/18836458/
A similar mechanism may also explain the observation of concomitant ANCA and anti-GBM disease in up to 30% of patients.
MPO has significant structural overlap with peroxidasin, a key enzyme in the crosslinking of collagen IV NC1 domain which harbor the anti-GBM cryptic epitope. When anti-peroxidasin antibodies develop, defective NC1 crosslinking may lead to exposure of the cryptic epitope leading to production of anti-GBM antibodies.
https://doi.org/10.1681/ASN.2018050519
However, not all ANCAs are pathogenic; in fact, many healthy controls are found to have autoantibodies against MPO and PR3. https://www.sciencedirect.com/science/article/pii/S0085253815545845
This may be because ANCAs targeting different epitopes have different pathogenicity, and/or that a secondary event leading to neutrophil activation is required for the disease to manifest.
In resting neutrophils, there is very little to no expression of ANCA antigen targets. Upon neutrophil activation. degranulation and apoptosis; however, these antigens become accessible to ANCA binding.
PR3 and MPO are also present in neutrophil extracellular traps (NETs) that form during neutrophil activation. Defective degradation of NETs could contribute to ANCA formation by prolonging exposure to these antigens.
The anti-thyroid drug, propylthiouracil (PTU) induces a change in MPO that results in abnormal NETs that are resistant to degradation. This may contribute to the breakdown of tolerance to MPO and result in autoantibody production. Indeed, ~30% of patients treated with PTU develop ANCAs.
https://pubmed.ncbi.nlm.nih.gov/12201217/
Other drugs that are associated with ANCA include hydralazine, penicillamine, allopurinol, and sulfasalazine, TNF-a inhibitors, among others. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4491282/
After ANCAs are formed, their binding to MPO or PR3 in primed neutrophils leads to excessive activation, with abnormal cytokine production, the release of ROS and lytic enzymes, as well as further NET formation, all of which injure endothelial cells.
PATHOLOGY OF ANCA-ASSOCIATED VASCULITIS
Lesions of ANCA-associated vasculitis begin with neutrophilic infiltration, leading to fibrinoid necrosis. Mononuclear cells subsequently surround and replace the neutrophils, leading to granuloma formation. Rarely, interstitial necrotizing lesions and granulomas, not associated with glomeruli or vessels may be seen in renal biopsy. Within glomeruli, the typical lesions are GBM rupture, segmental fibrinoid necrosis, and crescent formation.
ANCA vasculitis is characterized by repeated bouts of acute lesions, leading to the characteristic finding of both cellular, fibrous-cellular and fibrous crescents within the same biopsy.
The renal prognosis of patients with ANCA vasculitis is mostly dependent on the proportion of uninvolved (i.e., normal) glomeruli, and extent of scarring, which form the basis of the pathologic classification of ANCA-associated glomerulonephritis.
MANAGEMENT OF ANCA-ASSOCIATED VASCULITIS
The treatment of ANCA-associated vasculitis has undergone considerable review over the past two years, with the publication of major trials showing that a regimen fewer steroids was non-inferior to a standard-dose regimen (https://www.nejm.org/doi/full/10.1056/nejmoa1803537) and that even a steroid-free strategy, based on complement inhibition could be possible (https://www.nejm.org/doi/full/10.1056/nejmoa2023386).
For more details on ANCA-vasculitis management, please refer to:
Quick note: This post is to be used for informational purposes only and does not constitute medical or health advice. Each person should consult their own doctor with respect to matters referenced. Arkana Laboratories assumes no liability for actions taken in reliance upon the information contained herein.