Disease Week: Bacterial infection-associated glomerulonephritis and endocarditis-associated glomerulonephritis
Monday
Renal disease related to infective endocarditis was first reported over 100 years ago. However, the initial literature describing nephritis associated with infective endocarditis relied primarily on autopsy-based studies from the pre- and early post-antibiotic era.
Reviews from recent decades note the evolution in renal complications of infectious diseases. Demographics have changed from younger to older patients. The frequency of comorbidities including diabetes has increased.
Recent decades have seen a change in the infectious agents that cause renal disease, from primarily Streptococcal to a broader array of organisms compared to the past, with predominance of Staphylococci.
The historical division into subacute and acute bacterial endocarditis are no longer recommended in the current era and the term infective endocarditis is now preferred due to implication of other infectious organisms. [1] [2]
The largest biopsy-based clinicopathologic series on endocarditis-associated glomerulonephritis included 49 patients studied at two large nephropathology laboratories between 2001-2011; an expanded cohort to included 62 patients was published in 2017.
This data indicates that infective endocarditis-associated glomerulonephritis in the current era has significantly different clinical and pathologic features compared to those described historically.
Patients with postinfectious and infection-related glomerulonephritis typically present with acute nephritic syndrome and hypocomplementemia. In contrast, endocarditis-associated GN is unique in that nephritic syndrome is noted in <10% and acute renal failure in >80%.
Infective endocarditis is ongoing during the onset of the glomerulonephritis, with no latent period. Hence, the terms endocarditis-related or -associated GN are preferred over “postinfectious” which implies occurrence after eradication of the infection.
The concept of infection-related or -associated rather than postinfectious glomerulonephritis applies to other deep-seated or occult infections that may similarly be ongoing at the time of active renal disease.
Presentation with glomerulonephritis can even coincide with the first clinical recognition of infective endocarditis in some patients. [3] [4] [5]
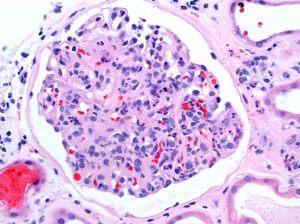
Tuesday
If you see the finding of a pauci-immune crescentic glomerulonephritis on renal biopsy, what is the first thing that comes to mind? Probably ANCA-related disease. However, this pattern can also be present in endocarditis-associated glomerulonephritis.
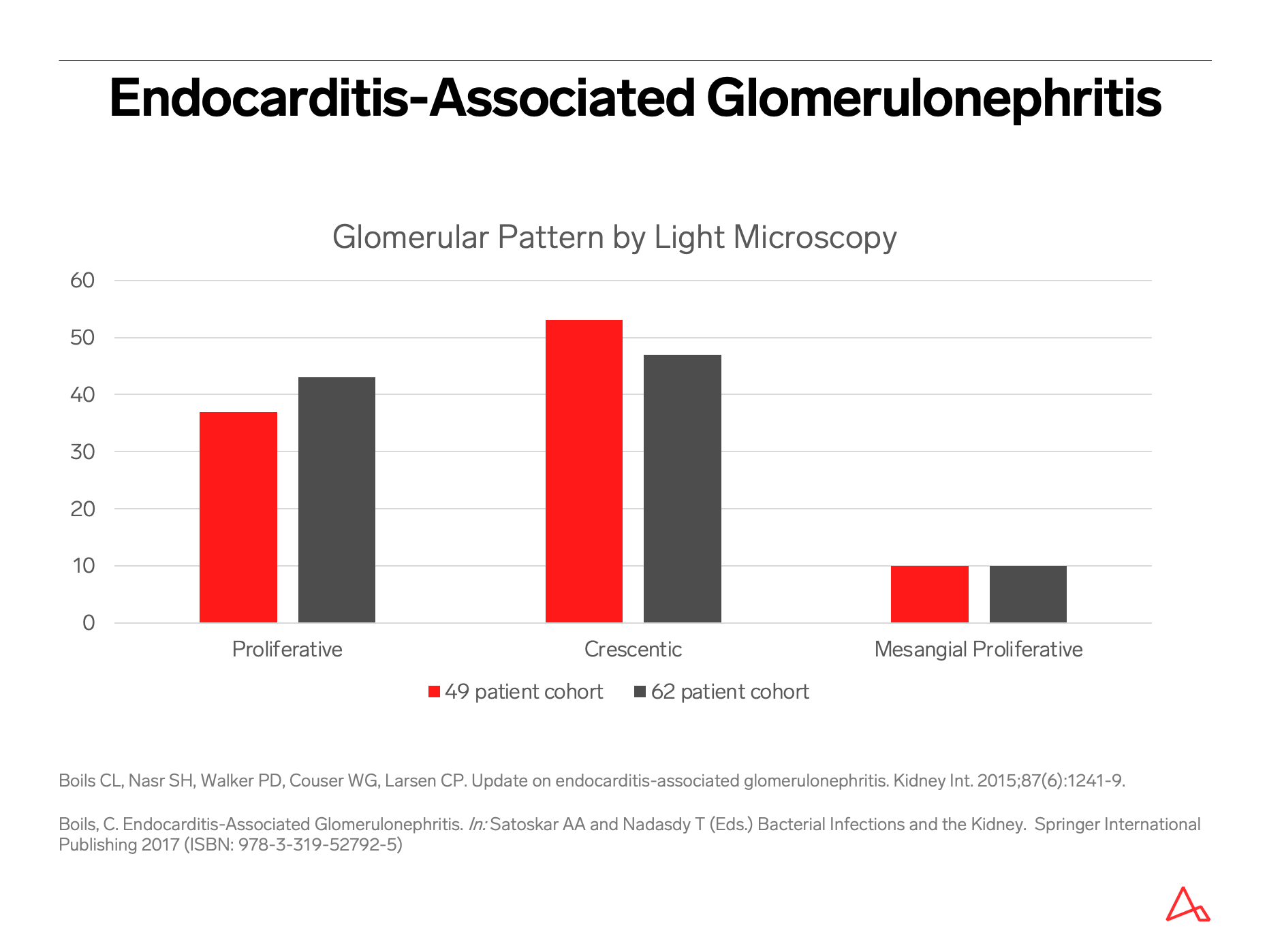
Endocarditis-associated glomerulonephritis challenges the classical “post-streptococcal glomerulonephritis” description of an infection-related immune complex-mediated endocapillary proliferative glomerulonephritis.
In fact, this classical post-streptococcal pattern represents the minority of glomerular disease related to infective endocarditis.
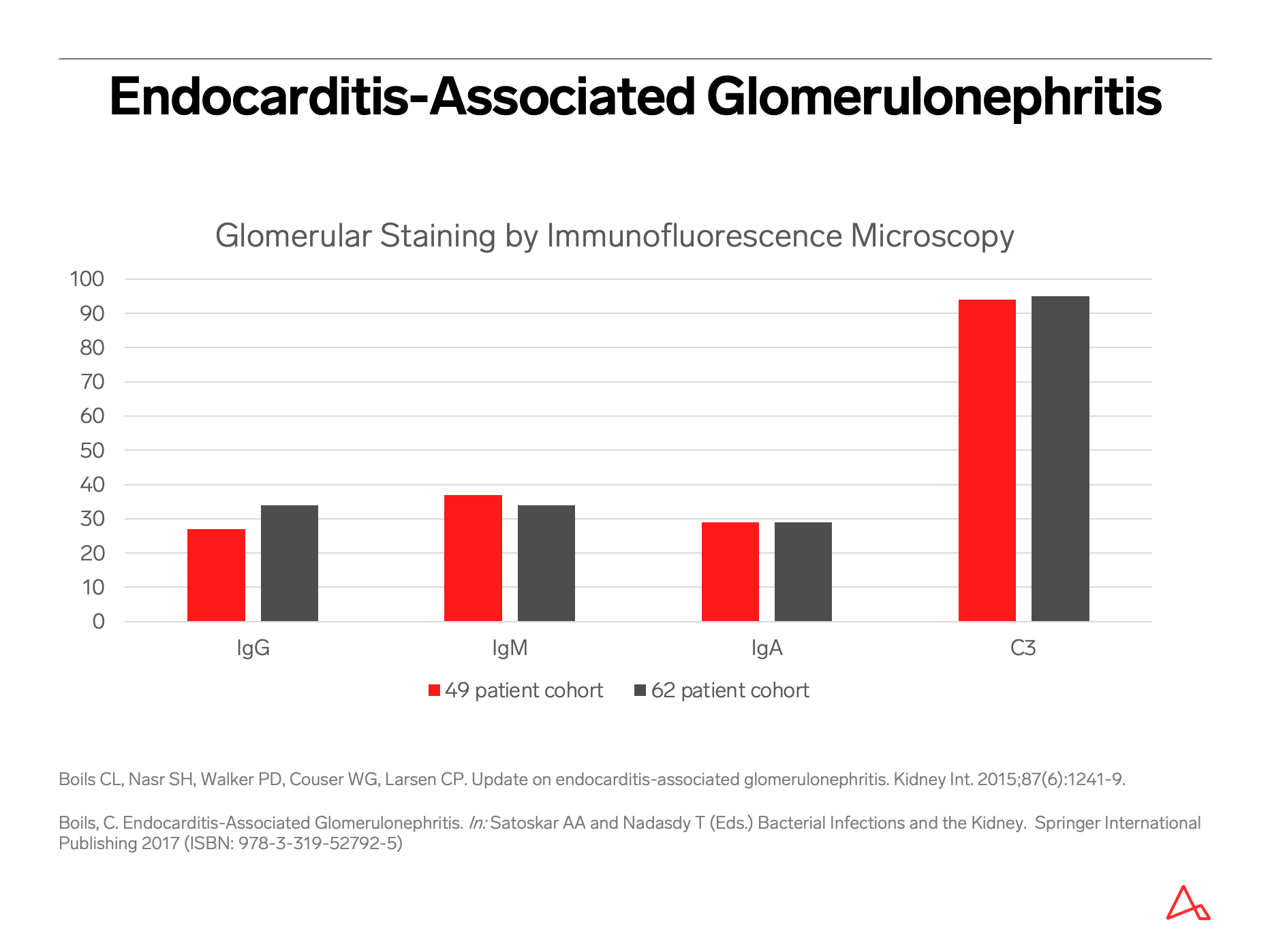
The most common pattern of glomerular injury in endocarditis-associated glomerulonephritis is a focal or diffuse crescentic glomerulonephritis. Typically, C3 is present, though often the lack of immunoglobulin qualifies glomerular disease as “pauci-immune.”
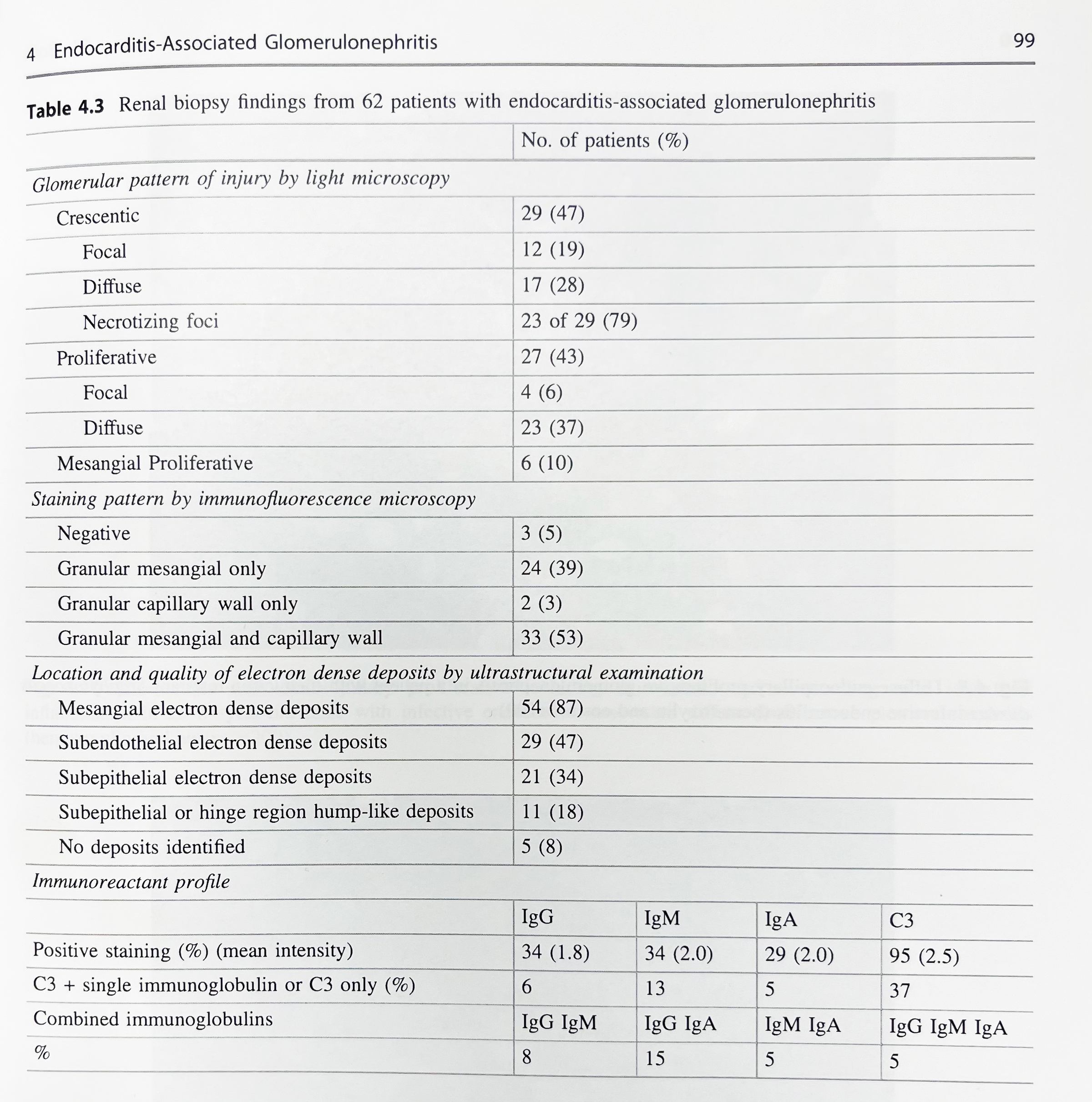
Renal biopsy clues for endocarditis-associated glomerulonephritis include strong C3 staining, and when immunoglobulins are present, IgM is the most common and most intensely positive.
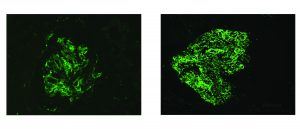
A series published in 2000 by Mujumdar et al. similarly found that two-thirds of patients with endocarditis-associated glomerulonephritis showed a pauci-immune crescentic pattern of glomerular injury. [6]
While there is a spectrum of morphologic patterns for endocarditis-associated glomerulonephritis, it is important to note that the most common pattern can mimic a disease process with a vastly different therapy and etiology!
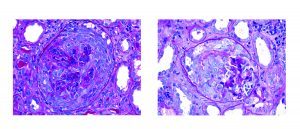
To make things even more challenging, not only is there morphologic overlap between endocarditis-associated GN and ANCA-related glomerulonephritis, there is also serologic overlap! 25% of patients with endocarditis-associated GN have a positive ANCA serology.
Perhaps this overlap makes since from a pathoetiological point of view. Infections have been implicated as triggers of ANCA activation. Furthermore, in ANCA-associated vasculitis, the complement system has been show to be activated.
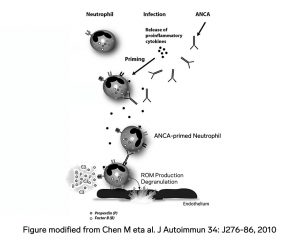
[7] [8]
Wednesday
The clinical diagnosis of endocarditis can be challenging. Clinicopathologic correlation and a strong index of suspicion is required by the pathologist and treating clinician alike!
One series reports that at the time of nephrology consult, infective endocarditis was unrecognized in almost 20% of cases. In another series, infective endocarditis was not recognized until autopsy in 38% of cases. [9]
Hypocomplementemia is absent in approximately 40% of patients with endocarditis-associated glomerulonephritis. Fever can also be absent. The prevalence of blood culture negative endocarditis occurs in 2.5% to 31% of patients. [10]
Quiz: What is the best imaging modality to diagnose infective endocarditis?
- Transthoracic echocardiography
- Transesophageal echocardiography
Answer: B. Transthoracic echocardiography cannot identify vegetations < 4 mm
[11]

[12]
Thursday
Staphylococcus infection-associated glomerulonephritis will often show IgA staining within glomeruli, therefore it is often referred to as IgA-dominant infection-associated GN, and may mimic primary IgA nephropathy. [13]
A subset of patients with IgA-dominant infection-associated GN will have concomitant leukocytoclastic vasculitis, which can mimic IgA vasculitis (formerly know as Henoch-Schönlein purpura). [14]
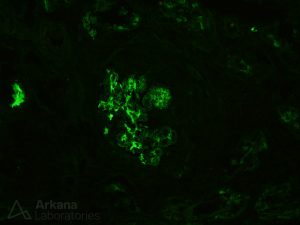
Age and diabetes mellitus have been found to be independent risk factors associated with an unfavorable prognosis for IgA-dominant infection-associated glomerulonephritis. [15] [16]
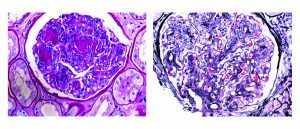
Despite the correlation between Staphylococcal infections and IgA staining, exceptions exist! In a study of 78 cases of staphylococcal infection-related GN, IgA staining was trace in 25%, mild in 19%, moderate in 44%, and strong in only 12% of the cases. [17]
Of similar interest, despite the prevalence of Staphylococcus as the infectious agent in 58% of patients with endocarditis-associated glomerulonephritis, IgA staining was only noted in 29% of cases. [18]
Friday
A paradigm shift has occurred in the epidemiology, bacteriology, and clinical and pathologic findings of infection-associated glomerulonephritis over the past few decades. The incidence of acute Streptococcal glomerulonephritis has declined, in part due to improved socioeconomic conditions and the advent of antibiotic therapy in developed countries. An increase in methicillin-resistant and sensitive Staphylococcal infections ranging from superficial skin infections to deep-seated occult infections such as visceral abscess, osteomyelitis, and endocarditis has been noted. This increase is seen in the expanding elderly population who have increasing comorbidities of diabetes and heart disease and increased use of cardiac devices, indwelling catheters and central lines.
It is also seen in the younger patient group who has seen a rise in intravenous drug use. These infections are usually active during the occurrence of glomerulonephritis, rather than occurring after an infection-free latent period as was seen in the past with prototypical post-streptococcal glomerulonephritis. This temporality introduces a difficult treatment dilemma for clinicians, where immunosuppression therapy to treat glomerulonephritis may be contraindicated in the context of an active infection.
Furthermore, the clinical diagnosis of infection can be challenging, and may coincide or even occur after the discovery of renal disease. The incidence of overt nephritic syndrome has decreased, and more often, acute renal failure, along with hematuria and proteinuria, is noted. In elderly patients, this clinical presentation may be attributed to worsening of underlying comorbidities of diabetes and heart disease rather than to infection. Fever may be absent. Blood cultures may be negative. Imaging studies may be required to identify the source of infection. Renal biopsy findings are heterogeneous and non-pathognomonic, and morphologic mimics are abundant.
Laboratory findings have exceptions as well. Complement levels can be normal. Positive ANCA serologies are noted in a subset of patients with infection-associated glomerulonephritis. It is therefore critical for the treating clinician and pathologist alike to be mindful of the changing landscape of infection-associated glomerulonephritis, inclusive of mimics and diagnostic pitfalls, and to maintain a high index of suspicion.
Despite these mimics and pitfalls, clinical features that might suggest the possibility of an infection-associated glomerulonephritis include risk factors for infection, such as diabetes, intravenous drug use, indwelling intravenous catheters, recent surgical or invasive procedure, prosthetic heart valve or other devices, poor dentition, or non-healing ulcers and surgical wounds. Hypocomplementemia, when present, should be noted. While a spectrum of morphologic patterns of glomerular injury can be noted by light, immunofluorescence and electron microscopy, certain biopsy features, or often the constellation of findings, can raise suspicion for infection. For example, despite the light microscopy findings, and despite presence or absence of immunoglobulins by immunofluorescence, strong C3 staining is typically noted in cases of infection-associated glomerulonephritis.
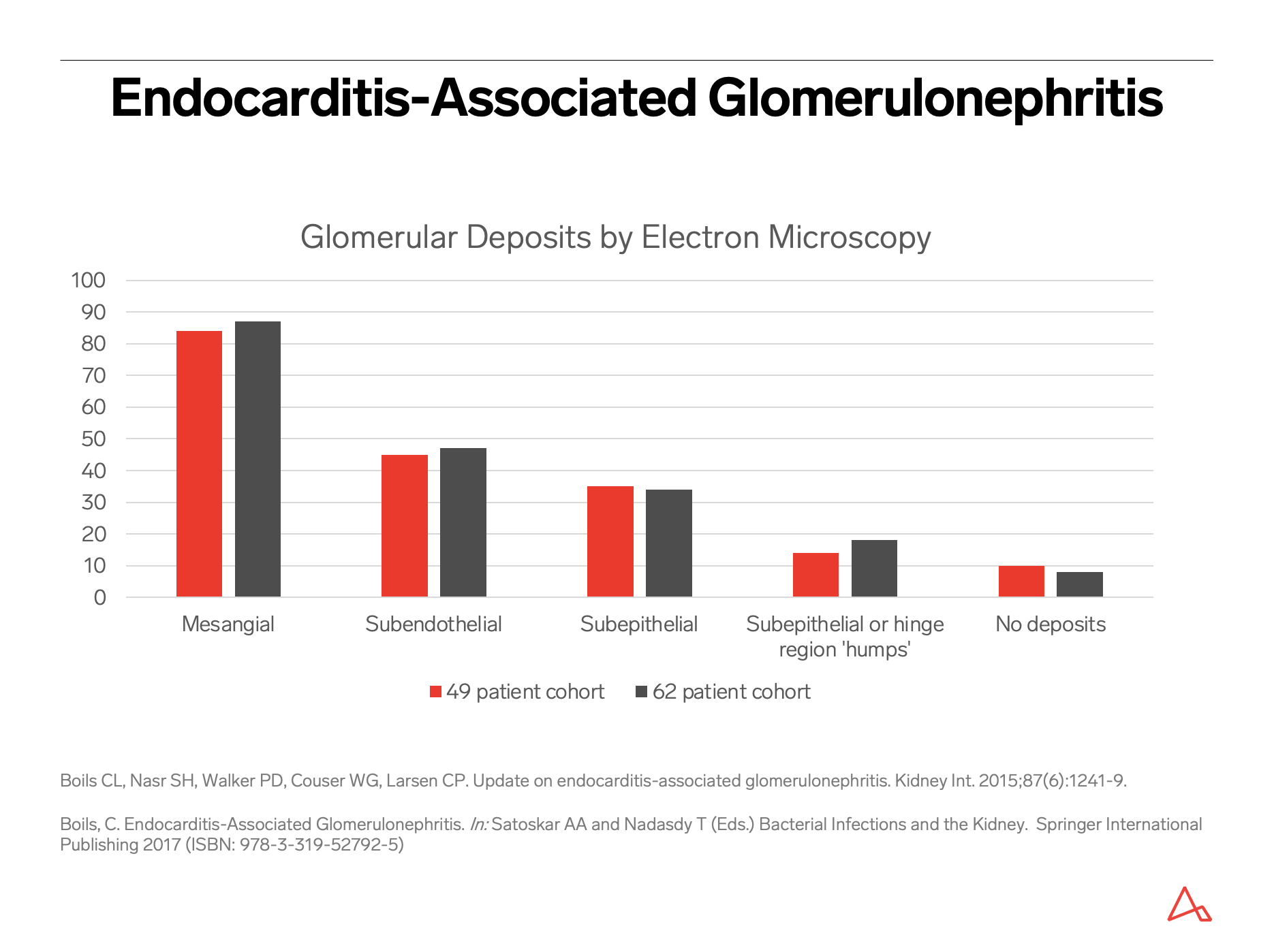
Presence of prominent subepithelial humps on electron microscopy, while not pathognomonic, should raise concern for possible infection; their absence, however, does not exclude infection. IgA nephropathy with presence of crescents and/or presence of endocapillary hypercellularity by neutrophils can raise the possibility of IgA-dominant infection-associated glomerulonephritis.
A crescentic glomerulonephritis by light microscopy, that otherwise looks like typical ANCA-related disease with presence of C3 or other immunoglobulins (especially IgM) that are of greater amount and intensity than typically seen in pauci-immune disease, together with electron-dense deposits by ultrastructural examination, should raise concern for infective endocarditis. Even for a pauci-immune crescentic glomerulonephritis, in the appropriate clinical context, underlying infection should be excluded as the etiology. Given the vastly different therapy required for this differential diagnosis, a thorough evaluation may be warranted.
https://www.ncbi.nlm.nih.gov/pubmed/31399725
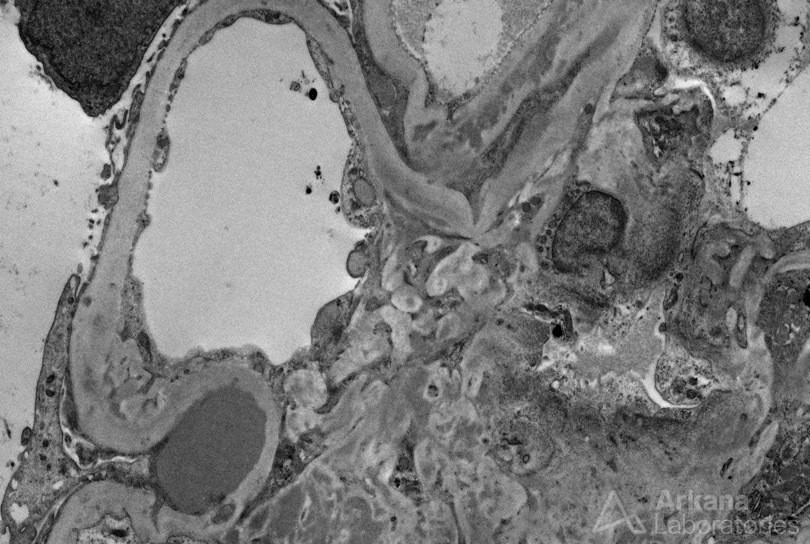
References:
[1] Ref: Nasr SH et al. Postinfectious glomerulonephritis in the elderly. J Am Soc Nephrol. 2011 Jan;22(1):187-95. doi: 10.1681/ASN.2010060611. Epub 2010 Nov 4. PMID: 21051737
[2] Ref: Nasr SH et al. Bacterial infection-related glomerulonephritis in adults. Kidney Int. 2013 May;83(5):792-803. doi: 10.1038/ki.2012.407. Epub 2013 Jan 9. PMID: 23302723
[3] Ref: Nasr SH et al. Acute postinfectious glomerulonephritis in the modern era: experience with 86 adults and review of the literature. Medicine (Baltimore). 2008 Jan;87(1):21-32. PMID:18204367
[4] Ref: Boils CL, Nasr SH, Walker PD, Couser WG, Larsen CP. Update on endocarditis-associated glomerulonephritis. Kidney Int. 2015;87(6):1241-9.
[5] Ref: Boils, C. Endocarditis-Associated Glomerulonephritis. In: Satoskar AA and Nadasdy T (Eds.) Bacterial Infections and the Kidney. Springer International Publishing 2017 (ISBN: 978-3-319-52792-5)
[6] Ref: Majumdar A, Chowdhary S, Ferreira MA et al. Renal pathological findings in infective endocarditis. Nephrol Dial Trans 2000; 15: 1782–1787.
[7] Ref: Chen M, Daha MR, Kallenberg CG. J Autoimmun. The complement system in systemic autoimmune disease. 2010 May;34(3):J276-86. doi: 10.1016/j.jaut.2009.11.014. Epub 2009 Dec 11. PMID:20005073
[8] Ref: Cartin-Ceba R, Peikert T, Specks u. Pathogenesis of ANCA-associated vasculitis. Curr Rheumatol Rep. 2012; 14 (6):481-93. PMID: 22927039
[9] Ref: Fernandez Guerrero ML, Alvarez B, Manzarbeitia F et al. Infective endocarditis at autopsy: a review of pathologic manifestations and clinical correlates. Medicine (Baltimore, MD) 2012; 91: 152–164.
[10] Ref: Fournier PE, Thuny F, Richet H et al. Comprehensive diagnostic strategy for blood culture-negative endocarditis: a prospective study of 819 new cases. Clin Infect Dis 2010; 51: 131–140.
[11] Ref: Cecchi EInfective endocarditis: transesophageal echocardiography in all or in selected cases? When is echocardiography highly predictive for complications? Ital Heart J. 2004 Sep;5(9):656-62 PMID:15568592
[12] Ref: Li JS et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000 Apr;30(4):633-8. Epub 2000 Apr 3. PMID:10770721
[13] Ref: Satoskar AA, Nadasdy G, at el. Staphylococcus infection-associated glomerulonephritis mimicking IgA nephropathy. CJASN 2006 November; 1(6):1179-86. PMID:17699345
[14] Satoskar AA et al. Henoch-Schönlein purpura-like presentation in IgA-dominant Staphylococcus infection – associated glomerulonephritis – a diagnostic pitfall. Clin Nephrol. 2013 Apr;79(4):302-12. doi: 10.5414/CN107756. PMID:23320971
[15] Ref: Bu R et al. Clinicopathologic features of IgA-dominant infection-associated glomerulonephritis: a pooled analysis of 78 cases.Am J Nephrol. 2015;41(2):98-106. doi: 10.1159/000377684. Epub 2015 Mar 3. PMID: 25765902
[16] Ref: Nasr SH et al. IgA-dominant acute poststaphylococcal glomerulonephritis complicating diabetic nephropathy. Hum Pathol. 2003 Dec;34(12):1235-41. PMID: 14691907
[17] Ref: Satoskar AA et al. Clin J Am Soc Nephrol. 2017 Jan 6;12(1):39-49. doi: 10.2215/CJN.05070516. Epub 2016 Nov 7. PMID: 27821389 Staphylococcus Infection-Associated GN – Spectrum of IgA Staining and Prevalence of ANCA in a Single-Center Cohort.
[18] Ref: Boils, C. Endocarditis-Associated Glomerulonephritis. In: Satoskar AA and Nadasdy T (Eds.) Bacterial Infections and the Kidney. Springer International Publishing 2017 (ISBN: 978-3-319-52792-5)
Quick note: This post is to be used for informational purposes only and does not constitute medical or health advice. Each person should consult their own doctor with respect to matters referenced. Arkana Laboratories assumes no liability for actions taken in reliance upon the information contained herein.