Monday
History of MG
Timeline of major discoveries in membranous glomerulopathy.
Heymann nephritis was unique as a model of membranous glomerulopathy when it was reported in 1959 because it was the first to produce renal disease through autosensitization. http://pediatrics.aappublications.org/content/7/5/691
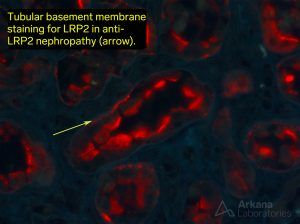
The antigenic target of Heymann nephritis (megalin) is largely absent from human podocytes but does have a role in human autoimmune disease as the antigenic target of anti-brush border antibody disease (LRP2-assoc nephropathy). https://jasn.asnjournals.org/content/29/2/644.long
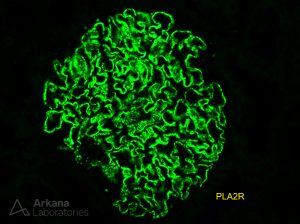
PLA2R was described as the major autoantigen of primary MG in 2009, approximately 50 years after the initial description of this form of glomerulonephritis. https://www.nejm.org/doi/full/10.1056/NEJMoa0810457
Tuesday
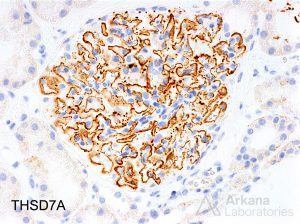
PLA2R and THSD7A
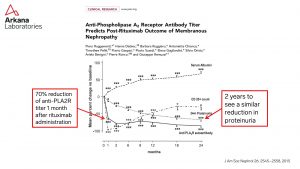
Monitoring for serum anti-PLA2R antibodies is useful to determine the prognosis at time of diagnosis, monitoring response to treatment, and predicting disease recurrence. https://jasn.asnjournals.org/content/jnephrol/26/10/2545.full.pdf
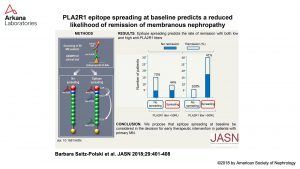
PLA2R epitope spreading: CysR only= “nonspreader” CysR + other (CTLD1, 5, 7, or 8) = “spreader”.
https://www.ncbi.nlm.nih.gov/pubmed/29114041
Tweet text: The prevalence of THSD7A positivity in patients with primary MG is approximately 2.5%.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5908687/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5628706/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5280014/
Wednesday
Pediatric MG
PLA2R shows positive staining in 45% (10/22) of pediatric primary membranous glomerulopathy. https://www.ncbi.nlm.nih.gov/pubmed/23903693
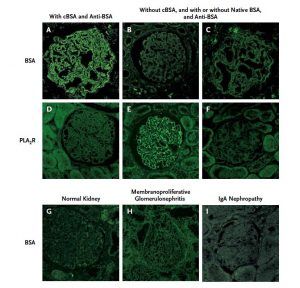
The case report of placental transfer of maternal antibodies directed against the neutral endopeptidase protein in fetal glomeruli was the first description of a podocyte antigen as the etiology of MG in humans. https://www.nejm.org/doi/full/10.1056/NEJMoa012895
Antibodies to BSA can cause a rare form of MG in young children that is important to detect as eliminating it from the diet could be beneficial. Could other food antigens be involved in the development of membranous nephropathy. https://www.nejm.org/doi/full/10.1056/nejmoa1013792
Thursday
Unusual membranous variants
20% of MG patients with light chain restriction have a lymphoproliferative disorder. Features worrisome for presence of underlying lymphoproliferative disorder on biopsy: absence of PLA2R, staining for single IgG subclass, presence of focal proliferation. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5733688/
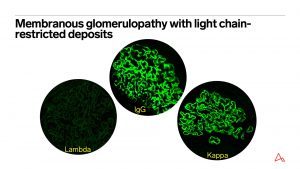
Membranous-like glomerulopathy with masked IgG Kappa deposits is a recently described form of glomerulonephritis that primarily affects young females. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5678740/
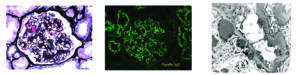
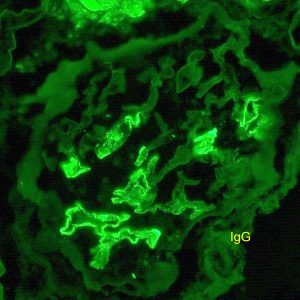
Segmental MG is more likely to have C1q and mesangial deposits than global MG. It does not simply represent an early form of MG as repeat biopsies continue to show segmental IgG. Arkana anecdotal experience is that it is PLA2R negative. https://cjasn.asnjournals.org/content/1/4/723
Friday
Membranous glomerulopathy: Looking forward
Many excellent reviews are available that detail the current state of knowledge in membranous glomerulopathy so I will not attempt to round out this #DiseaseWeek by writing another. Instead, I would like to paint, with broad brush strokes, what I believe to be the near future of diagnostics in membranous glomerulopathy and discuss the techniques of discovery being applied today that are likely to similarly impact other types of immune complex-mediated glomerulonephritis. We live in exciting times for discovery in medicine as the tools available today are so powerful that the molecular pathogenic mechanisms of disease are being worked out at a dizzying pace and membranous glomerulopathy is no exception.
Membranous glomerulopathy is a pattern of immune complex-mediated glomerulopathy in which the immune deposits are predominantly subepithelial and IgG is the principal immunoglobulin. Traditionally this disease has been divided into “primary” and “secondary”. Secondary associations are a broad and diverse group that includes drugs, malignancy, infection, and autoimmune disease, among others. (1) The renal biopsy diagnosis of a membranous glomerulopathy pattern of immune deposition provides a framework for the workup that should be initiated and also has prognostic and treatment implications. The discovery of PLA2R as the most common etiology of primary membranous glomerulopathy approximately 10 years ago ushered in a new era of diagnosis for this disease. (2) This discovery was rapidly applied to clinical practice as it became clear that this biomarker was useful for classifying disease, assessing disease activity, and prognostication. (3-5) The discovery of THSD7A followed shortly thereafter though it’s impact has not been as large due to the rarity of this type of membranous glomerulopathy. (6)
The horizon for membranous glomerulopathy looks promising for rapid discovery over the next ten years. Advances in mass spectrometry techniques to type membranous in tissue and whole proteome arrays for comprehensive screening of serum antibodies will enable unprecedented “fishing expeditions” and likely provide additional (if not all remaining) antigenic targets of membranous glomerulopathy antibodies. These newly discovered antigens can then be utilized as biomarkers for disease similar to PLA2R. Additionally, determination of the specific antigenic epitopes appears promising for subclassification of disease among patients with antibodies to the same protein. (7) This increasingly detailed understanding of membranous glomerulopathy on an antigenic level will revolutionize the diagnosis of all types of membranous similar to that which has already occurred for PLA2R. While not likely in the near term, I hope that by the end of my career in renal pathology, patients with membranous glomerulopathy will not only be diagnosed and followed based on a diagnostic assay specific for their disease, but that personalized treatment will exist for specific types of membranous glomerulopathy, yielding improved outcomes for these patients. This discovery process is going to be fun and I am glad to be practicing at this incredible moment in the history of medicine.
1. Glassock RJ. The pathogenesis of membranous nephropathy: evolution and revolution. Curr Opin Nephrol Hypertens 2012; 21: 235-242.
https://www.ncbi.nlm.nih.gov/pubmed/22388552
2. Beck LH, Bonegio RG, Lambeau G, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 2009; 361: 11-21.
https://www.ncbi.nlm.nih.gov/pubmed/19571279
3. Dahan K, Debiec H, Plaisier E, et al. Rituximab for Severe Membranous Nephropathy: A 6-Month Trial with Extended Follow-Up. J Am Soc Nephrol 2016; 28: 348-358.
https://www.ncbi.nlm.nih.gov/pubmed/27352623
4. Ruggenenti P, Debiec H, Ruggiero B, et al. Anti-phospholipase A2 receptor antibody titer predicts post-rituximab outcome of membranous nephropathy. J Am Soc Nephrol 2015; 26: 2545-2458.
https://www.ncbi.nlm.nih.gov/pubmed/25804280
5. Larsen CP, Messias NC, Silva FG, et al. Determination of primary versus secondary membranous glomerulopathy utilizing phospholipase A2 receptor staining in renal biopsies. Mod Pathol 2013; 26: 709-715.
https://www.ncbi.nlm.nih.gov/pubmed/23196797
6. Tomas NM, Beck LH, Jr., Meyer-Schwesinger C, et al. Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med 2014; 371: 2277-2287.
https://www.ncbi.nlm.nih.gov/pubmed/25394321
7. Seitz-Polski B, Debiec H, Rousseau A, et al. Phospholipase A2 Receptor 1 Epitope Spreading at Baseline Predicts Reduced Likelihood of Remission of Membranous Nephropathy. J Am Soc Nephrol 2017.
https://www.ncbi.nlm.nih.gov/pubmed/29114041
Quick note: This post is to be used for informational purposes only and does not constitute medical or health advice. Each person should consult their own doctor with respect to matters referenced. Arkana Laboratories assumes no liability for actions taken in reliance upon the information contained herein.



